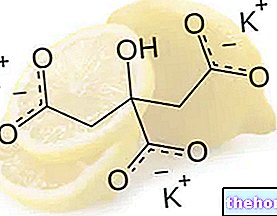
Potassium citrate (K3C6H5O7) is the potassium salt of citric acid (C6H8O7); in addition to the tripotassium form, potassium citrate can also be found in the dipotaxic (K2C6H6O7) and monopotassic (KC6H7O7) form.
At room temperature, it comes in the form of a white crystalline powder, slightly hygroscopic, odorless and with a saline taste. Both its constituents (potassium and citric acid) are abundantly present in nature and within the human organism:
- potassium taken from the diet is very important for the contraction of voluntary muscles, the heart and smooth muscles; moreover, it participates in the regulation of the acid-base balance and blood pressure. Many foods contain potassium, such as meat, some types of fish (such as salmon, cod and plaice), and in general all fruit, vegetables and legumes.
- Citric acid is a key molecule of the metabolic processes that take place inside every cell of the organism (Krebs cycle), and is present in important concentrations in the bones, with a stabilizing function. In addition to being produced by the organism, it abounds especially in citrus fruits, where it is also found in the form of potassium citrate: lemon juice contains 5-7% and "orange 1%", but it is found in fair concentrations in all fruit, in particularly in kiwis and strawberries.
Potassium citrate - or potassium citrate if you prefer - recognizes applications both in the food industry as an acidity corrector additive, and in the pharmaceutical one, as an alkalizing compound against metabolic, gastric and urinary acidosis; well known is for example the use of potassium citrate in the prevention of urinary stones caused by excess uric acid, cystine and xanthines.
Potassium citrate as a food additive
In the food industry, potassium citrate is used as a buffering agent, acidity regulator, metal ion chelator, and yeast nutrient in some fermented foods.
Potassium citrate in drugs and supplements
Potassium citrate has numerous medical and health applications:
- antacid: useful in case of gastric hyperacidity and heartburn, dyspepsia, digestive difficulties and pregnancy nausea; in fact, since it is a weak acid, in contact with the hydrochloric acid (strong acid) present in the stomach, in an environment characterized by a pH of 1.5-3, potassium citrate acts as a base by subtracting hydrogen ions from hydrochloric acid to give rise to potassium chloride and citric acid:
- K3C6H5O7 + HCl => K2C6H6O7 + KCl
K2C6H6O7 + HCl => KC6H7O7 + KCl
KC6H7O7 + HCl => C6H8O7 (citric acid) + KCl (potassium chloride) - potassium supplement: each gram of potassium citrate contains 383 mg of elemental potassium; in this regard, it should be borne in mind that the normally recommended total daily intake of potassium (diet + possible supplement) is around 4 grams.
Although significantly more expensive than other sources of potassium (for example potassium chloride), citrate is sometimes preferred for technological reasons (it lends itself to the preparation of effervescent products), health (see below) or for simple commercial appeal; - alkalizing agent: the intake of potassium citrate is able to increase the urinary pH, thus making the urine more alkaline; this intervention helps to prevent the precipitation of uric acid, cystine and xanthine crystals, making them more soluble in the urine. It is therefore an important help in people suffering from this type of urinary tract stones.
- The correction of the excessive acid load can be evaluated directly by measuring the urinary pH with suitable strips of litmus paper. The urinary pH varies during the day with more significant drops during the night and in the morning; for this reason it is preferable to measure it. especially upon awakening. If the urinary pH value is lower than 6 it is advisable, if confirmed by the doctor, to make any corrections by taking potassium citrate in the morning and in the evening, then verifying its urinary alkalizing action with the appropriate strips. presence of uratic calculi it is advisable to maintain the urinary pH between 6.5 and 6.8 to favor the dissolution of the uric acid crystals. A daily water intake of 2.5-3 liters is also recommended to facilitate the dilution of the acid urinary uric.
- The alkalizing action of potassium citrate is also exploited in patients suffering from metabolic acidosis (eg resulting from chronic renal failure or renal tubular acidosis) to increase blood pH, which is strictly regulated by the body in order to keep it within limits. very restricted. For the same reason, potassium citrate can also be taken to relieve urinary burns associated with minor urinary tract infections.
- prevention of metabolic acidosis in patients with kidney disease
Side effects and contraindications
Unless otherwise indicated by a doctor, potassium citrate should be swallowed with water (a glass of 250 ml) immediately after meals, in order to dampen the slightly corrosive effect on the teeth and on the mucosa of the upper digestive tract, which could also be associated with nausea. vomiting, diarrhea and stomach cramps. The assumption of potassium citrate together with water is also a stimulus for diuresis: the washing and diluting action of the urine is particularly useful in the presence of cystitis and a tendency to urinary stones.
Among the possible contraindications relating to the use of potassium citrate we mention:
renal failure, hyperkalemia, uncontrolled diabetes, gastric ulcer, adrenal insufficiency (e.g. Addison's disease), severe burns or other tissue lesions, dehydration, intake of potassium-sparing diuretic drugs (amiloride, spironolactone, triamterene)
Excess potassium can cause asthenia, muscle cramps, hypotension and bradycardia up to cardiac arrest.
In general, it is a good idea to get your doctor's approval before starting a treatment with potassium citrate on your own initiative, especially in the presence of:
kidney disease, congestive heart failure, hypertrophic cardiomyopathy, previous history of heart attack, other heart disease, diabetes, hypertension, gastric or intestinal obstruction, chronic diarrhea (such as that associated with ulcerative colitis or Crohn's disease);
taking drugs such as ACE inhibitors (eg enalapril), aldosterone blockers (eg eplerenone), or potassium-sparing diuretics (eg triamterene), because they could increase the side effects of potassium citrate, with negative effects especially at the level cardiac;
taking drugs such as aluminum salts (some antacids), anticholinergics (eg atropine), anorectics (eg phentermine) or certain stimulants (albuterol, amphetamine, pseudoephredine), due to the risk that their negative effects are enhanced by potassium citrate ;
intake of lithium or tetracyclines (eg Doxycycline), because their therapeutic effect could be enhanced by the intake of potassium citrate.





























