See also: ketogenic diet; diabetic ketoacidosis.
Generality
In the past it was thought that ketone bodies were due to excessive metabolism, caused by the ingestion of too much fat or diabetes. Ketone bodies, on the other hand, are naturally produced by our body: the brain adapts to use these metabolites in conditions of prolonged fasting (in diabetics, ketone bodies replace glucose metabolism) Furthermore, there can be an exasperation of the pathway of ketone bodies in case of poor nutrition.
What are the ketone bodies
Ketone bodies are derivatives of lipids (they derive from the metabolism of lipids, almost exclusively hepatic), but have characteristics that make them resemble sugars:
- High input speed;
- Quick to use.
Even some amino acids, in particular metabolic conditions, can originate ketone bodies (Leucine, Lysine, Phenylalanine, Isoleucine, Tryptophan and Tyrosine).
Biological role
- Ketone bodies are small in size, so they are transported very quickly (much more than fatty acids which, on the other hand, need transport proteins such as albumin);
- the ketone bodies are used almost exclusively by the muscles and peripheral tissues, but also by the heart (20-30% of the energy it uses comes from the ketone bodies) and by the brain (in case of prolonged fasting).
Synthesis
Ketone bodies are synthesized from acetyl coenzyme A, which derives from the metabolism of fatty acids.
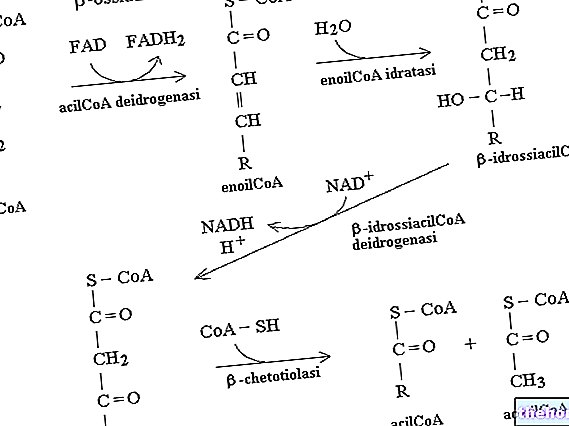
The enzyme that catalyzes the first stage is the Β-ketothiolase, which exploits the sulfur of acetyl coenzyme A to produce a Β-keto acyl-coenzyme A (it is the opposite reaction to that seen in the Β-oxidation of fatty acids); this reaction is not spontaneous but is driven by the subsequent reaction, catalyzed from "hydroxymethyl glutaryl coenzyme A synthase and which involves the attachment of a second acetyl coenzyme A, obtaining 3-hydroxy 3-methyl glutaryl coenzyme A.
Subsequently, a lytic enzyme intervenes which converts 3-hydroxy 3-methyl glutaryl coenzyme A into vinegar acetate which is a ketone body. The vinegar acetate can be sent to the peripheral tissues or, by the action of the enzyme hydroxy butyrate dehydrogenase, converted to 3-Β-hydroxy butyrate. If the vinegar acetate is in very high concentration, it can also spontaneously decarboxylate to acetone.
Acetone, vinegar acetate and 3-Β-hydroxy butyrate are the three ketone bodies that we consider; acetone is a waste product, which is produced randomly in the pathway of the ketone bodies and is expelled by exhalation and transpiration.
Use in peripheral tissues
Ketone bodies, produced in the liver, are sent to peripheral tissues.
Let's see, now what happens when the vinegar acetate and the 3-Β-hydroxy butyrate reach the peripheral tissues. The vinegar acetate is a Β-keto acid, therefore, if activated, it can be used in the Β-oxidation process to produce acetyl coenzyme A: therefore, it is necessary to transform a Β-keto acid into a Β-keto acyl coenzyme A.
When the vinegar acetate arrives in the mitochondrion of a cell of a peripheral tissue, it is subjected to the action of the enzyme succinyl coenzyme A transferase: through this enzyme, vinegar acetate reacts with succinyl coenzyme A (coming from the krebs cycle) and succinate and vinegar acetyl coenzyme A are obtained.
By exploiting succinyl coenzyme A, to activate the vinegar acetate, we jump into the krebs cycle, the stage that produces a GTP: this is the process, in terms of energy, that the cell is willing to pay to obtain the acetyl vinegar coenzyme A; the latter then goes under the action of Β-keto thiolase (Β-oxidation enzyme) to produce two molecules of acetyl coenzyme A which are sent to the krebs cycle.
If 3-Β-hydroxy butyrate is sent to the peripheral tissues, the latter, inside the mitochondrion, is converted into vinegar acetone by the action of the Β-hydroxy butyrate dehydrogenase enzyme, with the production of a NADH which corresponds to about 2.5 ATP; the vinegar acetate produced follows the path previously described.
The cell of a peripheral tissue draws more energy from 3-Β-hydroxy butyrate rather than from vinegar acetate, but the delivery of one or the other to the peripheral tissues depends on the energy availability of the liver.
C "is a non-negligible quantity of metabolized fatty acids, contained in peroxisomes and not in mitochondria; peroxisomes are organelles smaller than mitochondria and rich in metal ions and peroxidase enzymes. Peroxidase enzymes use hydrogen peroxide to promote redox processes, therefore in peroxisomes there is an enzymatic system capable of producing hydrogen peroxide.
In the Β-oxidation in peroxisomes, the "acyl coenzyme A, is obtained by the action of"acyl coenzyme A oxidase (In the mitochondria, on the other hand, the enzyme acyl coenzyme A dehydrogenase acted). Also in this case, the trans 2,3 enoyl coenzyme A is formed, which undergoes the action of a bifunctional enzyme (it performs the same function as in mitochondria by "enoyl coenzyme A hydratase and L-Β-hydroxy acyl coenzyme A dehydrogenase) and is thus converted into Β-keto acyl coenzyme A. This last, as in mitochondria, undergoes the action of Β-keto thiolase and acetyl coenzyme A and an acyl coenzyme A are obtained with a carbonaceous skeleton reduced by two units compared to the starting one, which returns to the circulation.