PLEASE NOTE: MEDICINAL PRODUCT NO LONGER AUTHORIZED
What is PhotoBarr?
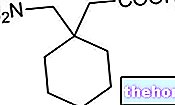
PhotoBarr is a powder that is made up into a solution for injection. Contains the active ingredient sodium porphyry.
What is PhotoBarr used for?
PhotoBarr is used in photodynamic therapy (therapy that uses light) for the ablation (removal) of high-grade dysplasia (abnormal cells at high risk of turning into cancer) in patients with Barrett's esophagus. This disease is characterized by a "alteration of the mucosa in the last section of the esophagus due to damage caused by" acid from the stomach.
Because the number of patients with Barrett's esophagus is low, the disease is considered 'rare' and PhotoBarr was designated an 'orphan medicine' (a medicine used in rare diseases) on 6 March 2002.
The medicine can only be obtained with a prescription.
How do you use PhotoBarr?
Photodynamic therapy with PhotoBarr must be performed or supervised by a physician experienced in endoscopic procedures (the endoscope is a thin tube used for observation inside the body) with laser, who has received adequate training in photodynamic therapy .
PhotoBarr treatment is a two-step process: the drug is first administered and then activated using a laser. PhotoBarr is given as a slow, careful intravenous injection, lasting 3-5 minutes, at a dose of 2 mg per kilogram of body weight. Approximately two days later, dysplasia and small areas of normal tissue around and below are brightened with light emitted by a specific wavelength laser, using a fiber optic cable through an endoscope. The type of instrument used, as well as the duration of the illumination, depend on the size of the area affected by the disease. If necessary, patients can undergo a second shorter treatment 2-3 days later. It is possible to repeat the cycle (one injection and one or two laser treatments) two more times, with an interval of at least three months, provided that the risk of narrowing of the esophagus is taken into account.
PhotoBarr should not be used in patients under the age of 18 due to the lack of information on the safety and efficacy of the medicine in this category.
Once PhotoBarr is administered, a special card containing a summary of the safety information for the medicinal product must be provided.
How does PhotoBarr work?
The active ingredient contained in PhotoBarr, the sodium porphyry, is a photosensitizing agent (substance that changes when exposed to light). After the injection of PhotoBarr, the porphimer is absorbed into the cells of the whole body. Subsequently, when illuminated with laser light of a specific wavelength, the porphimer is activated and reacts with the oxygen present in the cells, creating a type of highly reactive and toxic oxygen, called "singlet oxygen" (a free radical), which kills cells by reacting with their components, such as proteins and DNA, and destroying them. By restricting the lighting to the area of dysplasia, only the cells are damaged of this area, without affecting other parts of the organism.
How has PhotoBarr been studied?
PhotoBarr was studied in one main study involving 208 Barrett's esophagus patients with high-grade dysplasia. The effects of photodynamic therapy with PhotoBarr, used in combination with omeprazole (antacid medicine), were compared with those of omeprazole alone. The main measure of effectiveness was the number of patients who no longer had high-grade dysplasia at least six months after the first course of treatment. Patients were observed for at least two years.
What benefit has PhotoBarr shown during the studies?
Photodynamic therapy with PhotoBarr added to omeprazole treatment resulted in an increase in the number of patients whose dysplasia has been eliminated. After six months, 72% of patients treated with PhotoBarr in combination with omeprazole no longer had signs of high-grade dysplasia, compared with 31% of those who had taken omeprazole alone. Similar results were observed in the two groups after two years.
What is the risk associated with PhotoBarr?
The most common side effects with PhotoBarr (seen in more than 1 in 10 patients) are dehydration, esophageal stricture (narrowing of the esophagus), vomiting, dysphagia (difficulty swallowing), constipation, nausea, photosensitivity reactions (sunburn-like reactions) ) and pyrexia (fever). Because laser treatment causes swallowing difficulties, including pain, nausea and vomiting, patients should only take liquid foods for a few days after the course of treatment, in some cases up to four weeks. full list of side effects reported with PhotoBarr, see package leaflet.
PhotoBarr must not be used in people who may be hypersensitive (allergic) to sodium porphyrins and other porphyrins or any of the other ingredients. PhotoBarr should not be used in patients with porphyria (inability to metabolize porphyrins), severe kidney or liver failure, esophageal or gastric varices (swollen veins), large esophageal ulcers, fistulas (abnormal openings) between the esophagus and trachea or bronchi or suspected erosion of major blood vessels.
All patients taking PhotoBarr develop increased sensitivity to light and therefore should avoid exposing their skin and eyes to very bright light for at least three months after injection. See the package leaflet for further details.
Why has PhotoBarr been approved?
The Committee for Medicinal Products for Human Use (CHMP) decided that PhotoBarr's benefits are greater than its risks in photodynamic therapy for the ablation of high-grade dysplasia in patients with Barrett's esophagus. The Committee recommended that it be released. "Marketing Authorization" for PhotoBarr.
What measures are being taken to ensure the safe use of PhotoBarr?
The company that makes PhotoBarr is preparing information material in agreement with the authorities of the Member States on the subject of legislation on medicines. This will ensure that all doctors who prescribe the drug and all pharmacists who sell it are provided with information packs for healthcare professionals and patients.These packages will contain information about PhotoBarr and how to use it safely.
More information about PhotoBarr:
On 25 March 2004, the European Commission issued Axcan Pharma International B.V. a "Marketing Authorization" for PhotoBarr, valid throughout the European Union. The Marketing Authorization was renewed on March 25, 2009.
For the summary of the opinion of the Committee for Orphan Medicinal Products on PhotoBarr click here
For the full version of PhotoBarr's EPAR click here.
Last update of this summary: 03-2009.
The information on PhotoBarr - sodium porphyry published on this page may be out of date or incomplete. For a correct use of this information, see the Disclaimer and useful information page.