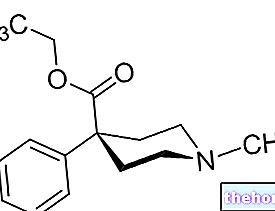
LYSEEN ® is a medicinal product based on Pridinol mesylate
THERAPEUTIC GROUP: Muscle relaxants with central and peripheral action

Indications LYSEEN ® - Pridinol
LYSEEN ® is indicated in the treatment of muscle pain, contractures of central and peripheral origin, lumbago and torticollis.
Mechanism of action LYSEEN ® - Pridinol
LYSEEN ® is a medicinal product based on Pridinol mesylate, an active ingredient derived from piperidinpropyl alcohol, and with both central and peripheral muscle relaxant activity.
From a molecular point of view, Pridinol exerts an atropine-similar action at the level of smooth and striated muscle, interacting and blocking the muscarinic receptors expressed by the effector organs innervated by post-ganglion fibers, thus exerting a peripheral spasmolytic and decidedly muscle relaxant action.
Studies show how this molecule can also act at the level of the central nervous system, inhibiting some motor centers involved in the regulation of muscle tone, thus determining a centrally acting myorelaxant activity.
Following oral or parenteral administration, Pridinol mesylate is rapidly absorbed, reaching the maximum plasma concentration within the first hour, biodistributing uniformly in the organism, to be subsequently excreted sulfurate and glucuronate mainly by the kidney.
Studies carried out and clinical efficacy
LYSEEN IN THE TREATMENT OF SPASTIC PARALYSIS IN PATIENTS WITH MENTAL DISORDERS
Przegl Lek. 1969 Apr 14; 25: 375-6.
[Lyseen in the treatment of cildren with mental disorders complicated by spastic paralysis].
Kwiatkowska E, Rosa-Garlicka Z.
In light of the modalities of action of Pridinol, this study seeks to characterize its clinical efficacy even in small patients with mental disorders complicated by spastic paralysis.
LYSEEN IN THE TREATMENT OF VERTEBRAL SYNDROME
Schweiz Rundsch Med Prax. 1975 Nov 4; 64: 1423-4.
[The treatment of vertebral syndromes with nova-lyseen (author "s transl)].
Müller P, Kunz W.
Very old German study that lays the foundations of the treatment with Pridinol in the vertebral syndrome, enjoying, among other things, also a good therapeutic success.
PRIDINOL MESILATE AND MUSCLE PAIN
Boll Chim Farm. 1985 Aug; 124: 102S-107S.
[Comparison between imidazole-2-hydroxybenzoate and prindinole mesylate in the treatment of post-traumatic muscular pain].
Monteleone R, Foppiani E, Gini M, Schiavi M, Ciani D.
Old Italian study that tries to characterize the therapeutic activity of Pridinol mesylate by comparing it to that of imidazole 2 hydroxybenzoate in the treatment of post-traumatic muscle pain, with promising results.
Method of use and dosage
LYSEEN ®
Injectable solution for intramuscular use of 2 mg of Pridinol mesylate per vial;
Pridinol mesylate 4 mg tablets;
Suppositories of 6 mg of Pridinol Mesylate.
The choice of dosage, format and therapeutic scheme is up to the doctor after carefully evaluating the patient's general health status and the severity of his clinical picture.
Generally the attack phase, for which parenteral administration of the drug is recommended, can be followed by the maintenance phase with tablets and suppositories.
Different therapeutic schemes are instead recommended in the case of nocturnal cramps.
LYSEEN ® warnings - Pridinol
Therapy with LYSEEN ® must be preceded by a careful medical examination aimed at identifying the potential causes and origins of the pain complained of and the consequent prescribing appropriateness.
Medical supervision would also be necessary during treatment, in order to identify possible side effects early and therefore promptly remedy.
The effectiveness of therapy with LYSEEN ® is greater the earlier it is started.
The use of LYSEEN ® should be carried out with particular caution in patients suffering from renal insufficiency, impaired renal function and hypotension.
LYSEEN ® in tablets contains lactose, therefore not indicated in patients with lactase enzyme deficiency, glucose-galactose malabsorption syndrome and galactosemia.
The presence of parahydroxybenzoates in LYSEEN ® suppositories, on the other hand, could increase the risk of adverse hypersensitivity reactions, especially in atopic patients.
PREGNANCY AND BREASTFEEDING
The absence of studies able to better characterize the safety profile of Pridinol for the health of the fetus and the infant, extend the aforementioned contraindications to the use of LYSEEN ® also to pregnancy and the subsequent breastfeeding period.
Interactions
The simultaneous administration of anticholinergics could increase the biological effects of Pridinol.
Contraindications LYSEEN ® - Pridinol
The use of LYSEEN ® is contraindicated in patients suffering from glaucoma, prostatic hypertrophy, urinary retention syndromes or intestinal obstruction and tachyarrhythmia, in patients hypersensitive to the active substance or to one of its excipients and in pregnant and lactating women.
Undesirable Effects - Side Effects
Although the use of LYSEEN ® is generally safe and well tolerated, sometimes the intake of Pridinol could cause the onset of asthenia, dry mouth and very rarely dizziness and dizziness.
Even more rare are adverse reactions worthy of clinical note such as those due to hypersensitivity to the active substance.
Note
LYSEEN ® is a prescription drug.
The information on LYSEEN ® - Pridinol published on this page may be out of date or incomplete. For a correct use of this information, see the Disclaimer and useful information page.



.jpg)