BATRAFEN ® is a drug based on Ciclopirox
THERAPEUTIC GROUP: Antifungals for topical use

Indications BATRAFEN ® Ciclopirox
BATRAFEN ® is indicated in the treatment of cutaneous mycoses caused by fungi sensitive to Ciclopirox.
The enamel form is also indicated in the treatment of onychomycosis.
Mechanism of action BATRAFEN ® Ciclopirox
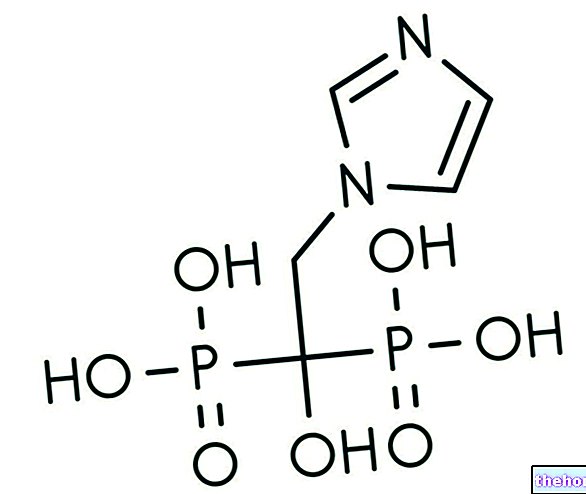
BATRAFEN ® is a drug based on Ciclopirox, an active ingredient with broad spectrum antifungal and antibacterial activity and therefore used successfully in the treatment of dermatomycosis and onychomycosis.
Applied topically, the drug concentrates on the skin level where it carries out its therapeutic activity by chelating different ions, including iron, thus removing them from fungal metabolism and inevitably compromising their metabolic and reproductive capacities.
All this takes concrete form in the static and fungicidal fungi action, which guarantees a prompt remission of the complained symptoms.
Several studies have also attributed to this active ingredient an anti-inflammatory potential, which could prove to be important in the management of the disease.
Studies carried out and clinical efficacy
THE CICLOPIROX IN THE TREATMENT OF THE "ONICOMICOS DELL" ALLUCE
An Bras Dermatol. 2012 Jan-Feb; 87: 19-25.
Comparative clinical evaluation of efficacy and safety of a formulation containing ciclopirox 8% in the form of a therapeutic nail lacquer in two different posologies for the treatment of onychomycosis of the toes.
Schalka S, Nunes S, Gomes Neto A.
Study which, re-evaluating the different dosage regimens suggested in the course of onychomycosis, reaffirms the clinical efficacy of Ciclopirox in the treatment of onychomycosis of the big toe
PHARMACOKINETIC CHARACTERISTICS OF CICLOPIROX-BASED ENAMEL
J Eur Acad Dermatol Venereol. 2013 Feb; 27: e153-8. doi: 10.1111 / j.1468-3083.2012.04529.x. Epub 2012 Mar 26.
Nail penetration and predicted mycological efficacy of an innovative hydrosoluble ciclopirox nail lacquer vs. a standard amorolfine lacquer in healthy subjects.
Monti D, Herranz U, Dal Bo L, Subissi A.
Interesting study that evaluates the penetration capacity of ciclopiroxil in the nails, considering the use of innovative pharmacological formats.
CYCLOPIROX AS AN ANTICANCER POTENTIAL
J Clin Pharm Ther. 2011 Apr; 36: 128-34. doi: 10.1111 / j.1365-2710.2010.01172.x. Epub 2010 Aug 24.
The repositioning of the anti-fungal agent ciclopirox olamine as a novel therapeutic agent for the treatment of haematologic malignancy.
Weir SJ, Patton L, Castle K, Rajewski L, Kasper J, Schimmer AD.
Very recent study which reaffirms the efficacy of systemic Ciclopirox as a potential antitumor agent, especially effective in the treatment of malignant haematological diseases.
Method of use and dosage
BATRAFEN ®
Cream, gel, cutaneous solution and cutaneous powder with 1% of Ciclopirox;
Nail polish with 8% of Ciclopirox.
Generally it is recommended to apply the appropriate amount of cream, skin solution or skin powder 2-3 times a day directly on the region affected by the infectious process, until the symptoms are completely resolved.
Generally it is recommended to extend the application of the drug for another 2 weeks from the remission of symptoms.
The correct use of the enamel, on the other hand, requires the application of the product on the affected nail, previously cleansed and cleared of the hyperkeratotic layers, once a day, until the symptoms are resolved.
Warnings BATRAFEN ® Ciclopirox
Although the use of BATRAFEN ® is generally safe and free from clinically relevant side effects, the application of this medicine should be accompanied by compliance with different health and hygiene regulations useful for optimizing the therapeutic efficacy of the treatment and limiting the potential effects. collateral.
The appropriate cleansing of the skin region to be treated and of the hands following the application of the product, would in fact allow to avoid the dispersion of the drug, thus limiting the onset of resistant strains.
The patient receiving BATRAFEN ® should avoid direct exposure to ultraviolet radiation, given the phototoxic potential of the active ingredient.
Prolonged use of Ciclopirox could lead to the onset of hypersensitivity reactions to the drug.
PREGNANCY AND BREASTFEEDING
The use of BATRAFEN ® during pregnancy and in the subsequent breastfeeding period should be defined by the physician according to the clinical needs of the patient and supervised throughout the duration of treatment.
Interactions
No drug interactions worthy of clinical note are currently known.
Contraindications BATRAFEN ® Ciclopirox
The use of BATRAFEN ® is contraindicated in patients hypersensitive to the active substance or to one of its excipients.
Undesirable Effects - Side Effects
The use of BATRAFEN ® is generally safe and well tolerated.
Only rarely have transient local reactions such as itching, burning and skin irritation been observed.
Note
BATRAFEN ® is a drug subject to mandatory medical prescription.
The information on BATRAFEN ® Ciclopirox published on this page may be out of date or incomplete. For a correct use of this information, see the Disclaimer and useful information page.




.jpg)





















