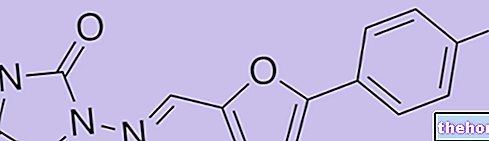
AZYTER ® is an Azithromycin-based drug
THERAPEUTIC GROUP:
Antibacterials - Antibiotics

Indications AZYTER ® Azithromycin
AZYTER ® is indicated as an antibiotic in the topical treatment of bacterial conjunctivitis caused by sensitive microorganisms and by Chlamydia trachomatis.
Mechanism of action AZYTER ® Azithromycin
AZYTER ® is an antibiotic in eye drops capable of carrying out its therapeutic action topically, without being absorbed systemically.
Bacteriostatic antimicrobial activity is essentially linked to the ability to inhibit protein synthesis, binding the 50S ribosomal subunit and thus preventing elongation of the nascent peptide chain.
The consequent lack of structural proteins and enzymes prevents the microorganism from carrying out its biosynthetic activities.
Despite the good therapeutic efficacy, proved to be such against both Gram positive and Gram negative cocci and bacilli, often different microorganisms resist azithromycin therapy thanks to the development of different resistance mechanisms characterized by:
- Expression of efflux pumps that prevent the antibiotic from reaching effective concentrations inside the cell;
- Variation of the biological target of the therapy;
- Reduction of membrane permeability.
Studies carried out and clinical efficacy
1. L "AZITHROMYCIN IN THE TREATMENT OF CHRONIC BLEPHARITIS
Arq Bras Oftalmol. 2012 May-Jun; 75: 178-82.
Azithromycin 1.5% ophthalmic solution: efficacy and treatment modalities in chronic blepharitis.
Fadlallah A, Rami HE, Fahd D, Dunia I, Bejjani R, Chlela E, Waked N, Jabbour E, Fahed S.
Work that demonstrates how the use of azithromycin-based ophthalmic solution can be an effective treatment in chronic blepharitis.
In these cases, treatments that were also significantly longer than that commonly envisaged were used.
2 . AZITHROMYCIN IN EYE DROPS IN THE POST-SURGICAL PHASES
J Ocul Pharmacol Ther. 2012 Aug; 28: 428-32. Epub 2012 Mar 15.
A comparison of azithromycin and tobramycin eye drops on epithelial wound healing and tolerance after penetrating keratoplasty.
Blavin J, Sauer A, Saleh M, Gaucher D, Speeg-Schatz C, Bourcier T.
Study that demonstrates the good tolerability of the "ocular epithelium" to Azithromycin even after keratoplasty surgery, thus allowing Azithromycin to be included in the possible antibiotics useful in the post-surgical phases.
3. AZITROMYCIN IN THE TREATMENT OF CONJUNCTIVITIS
Drugs. 2012 Feb 12; 72: 361-73.
Azithromycin 1.5% ophthalmic solution: in purulent bacterial or trachomatous conjunctivitis.
Garnock-Jones KP.
Trial that demonstrates the excellent efficacy and good tolerability of Azithromycin in ocular solution in the treatment of bacterial conjunctivitis even when sustained by Chlamydia Trachomatis
Method of use and dosage
AZYTER ®
Eye drops in single-dose container of 250 mg of solution containing 3.75 mg of Azithromycin dihydrate.
The therapeutic procedure generally involves the instillation of a drop in the conjunctival fornix twice a day for three days.
In order to optimize the therapeutic efficacy, it would be advisable to thoroughly cleanse the hands immediately before and after using AZYTER ® and not to touch the eye with the tip of the container.
Containers must be disposed of after use.
AZYTER ® Azithromycin warnings
AZYTER ® based therapy must be prescribed by a physician experienced in the treatment of ocular infections, given the need in some cases to resort to the use of systemic therapies.
If signs and symptoms of allergic manifestations appear, the patient, after contacting his doctor, should consider the need to stop the current therapy.
The maximum duration of therapy is three days, so it would be advisable to review it if results are late in coming.
The patient treated with AZYTER ® must not use contact lenses during the therapeutic process.
PREGNANCY AND BREASTFEEDING
The absence of studies able to reveal the safety of the drug on the accidentally exposed fetus considerably limit the use of AZYTER ® during pregnancy.
The low systemic absorption of Azithromycin in this case makes it possible to use the drug during breastfeeding.
Interactions
The low systemic absorption makes the administration of AZYTER ® safe.
However, it would be preferable to take a second eye drop by at least 15 minutes.
Contraindications AZYTER ® Azithromycin
AZYTER ® is contraindicated in patients hypersensitive to Azithromycin and its excipients.
Undesirable Effects - Side Effects
AZYTER ® therapy exposes the patient to clinically insignificant local side effects such as burning and discomfort after instillation and blurred vision.
Only rarely has increased lacrimation and hypersensitivity to the drug been observed.
Note
AZYTER ® is a prescription drug.
The information on AZYTER ® Azithromycin published on this page may be out of date or incomplete. For a correct use of this information, see the Disclaimer and useful information page.