Generality
Nitrogen bases are aromatic heterocyclic organic compounds, containing nitrogen atoms, which take part in the constitution of nucleotides.
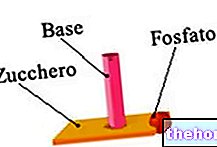
Fruit of the union of a nitrogenous base, a pentose (ie a sugar with 5 carbon atoms) and a phosphate group, nucleotides are the molecular units that make up the nucleic acids DNA and RNA.
In DNA, the nitrogenous bases are: adenine, guanine, cytosine and thymine; in "RNA, they are the same, except thymine, in place of which c" is a nitrogenous base called uracil.
Unlike those of RNA, the nitrogenous bases of DNA form pairing or base pairs. The presence of such pairing is possible because DNA has a double-stranded structure of nucleotides.
The gene expression depends on the sequence of nitrogenous bases joined to the DNA nucleotides.
What are nitrogenous bases?
Nitrogenous bases are the organic molecules, containing nitrogen, which take part in the constitution of nucleotides.
Formed each of a nitrogenous base, a 5-carbon sugar (pentose) and a phosphate group, nucleotides are the molecular units that make up the nucleic acids DNA and RNA.
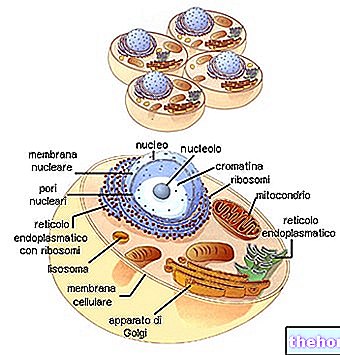
The nucleic acids DNA and RNA are the biological macromolecules, on which the development and proper functioning of the cells of a living being depend.
THE NITROGEN BASES OF NUCLEIC ACIDS
The nitrogenous bases that make up the DNA and RNA nucleic acids are: adenine, guanine, cytosine, thymine and uracil.
Adenine, guanine and cytosine are common to both nucleic acids, ie they are part of both DNA nucleotides and RNA nucleotides. Thymine is exclusive to DNA, while uracil is exclusive to RNA.
Making a brief summary, therefore, the nitrogenous bases that form a nucleic acid (be it DNA or RNA) belong to 4 different types.

ABRREVIATIONS OF NITROGEN BASES
Chemists and biologists have considered it appropriate to abbreviate the names of the nitrogenous bases with a single letter of the alphabet. In this way, they have made the representation and description, on texts, of nucleic acids easier and faster.
L "adenine coincides with the capital letter A; guanine with the capital letter G; cytosine with the capital letter C; thymine with the capital letter T; finally, l" uracil with the capital letter U.
Classes and structure
There are two classes of nitrogenous bases: the class of nitrogenous bases that derive from pyrimidine and the class of nitrogenous bases that derive from purine.

Figure: generic chemical structure of a pyrimidine and a purine.
The nitrogenous bases that derive from pyrimidine are also known by the alternative names of: pyrimidine or pyrimidine nitrogenous bases; while the nitrogenous bases that derive from purine are also known with the alternative terms of: purine or purine nitrogenous bases.
Cytosine, thymine and uracil belong to the class of pyrimidine nitrogenous bases; adenine and guanine, on the other hand, make up the class of purine nitrogenous bases.
Examples of purine derivatives, other than the nitrogenous bases of DNA and RNA
Among the purine derivatives, there are also organic compounds that are not nitrogenous bases of DNA and RNA. For example, compounds such as caffeine, xanthine, hypoxanthine, theobromine and uric acid fall into the above category.
WHAT ARE NITROGEN BASES FROM THE CHEMICAL POINT OF VIEW?
Organic chemists define nitrogenous bases and all derivatives of purine and pyrimidine as aromatic heterocyclic compounds.
- A heterocyclic compound is an organic ring (or cyclic) compound which, in the aforementioned ring, has one or more atoms other than carbon. In the case of purines and pyrimidines, the atoms other than carbon are the nitrogen atoms.
- An aromatic compound is an organic ring compound having structural and functional characteristics similar to those of benzene.
STRUCTURE

Figure: chemical structure of benzene.
The chemical structure of the nitrogenous bases derived from pyrimidine consists mainly of a single ring with 6 atoms, 4 of which are carbon and 2 of which are nitrogen.
In fact, a pyrimidine nitrogen base is a pyrimidine with one or more substituents (ie a single atom or group of atoms) bonded to one of the carbon atoms of the ring.
On the other hand, the chemical structure of nitrogenous bases derived from purine consists mainly of a double ring with 9 total atoms, 5 of which are carbon and 4 of which are nitrogen. The aforementioned double ring with 9 total atoms derives from the fusion of a pyridiminic ring (ie the pyrimidine ring) with an imidazole ring (ie the imidazole ring, another heterocyclic organic compound).

Figure: structure of imidazole.
As is known, the pyrimidine ring contains 6 atoms; while the imidazole ring contains 5. With the fusion, the two rings put in common two carbon atoms each and this explains why the final structure contains, specifically, 9 atoms.
POSITION OF NITROGEN ATOMS IN PURINS AND PYRIMIDINS
To simplify the study and description of organic molecules, organic chemists have thought of assigning an identification number to the carbons and to all the other atoms of the supporting structures. The numbering always starts from 1, is based on very specific assignment criteria (which, here, it is better to leave out) and serves to establish the position of each atom within the molecule.
For pyrimidines, the numerical assignment criteria establish that the 2 nitrogen atoms occupy position 1 and position 3, while the 4 carbon atoms reside in position 2, 4, 5 and 6.
For purines, on the other hand, the numerical assignment criteria establish that the 4 nitrogen atoms occupy the position 1, 3, 7 and 9, while the 5 carbon atoms reside in position 2, 4, 5, 6 and 8.
Position in the nucleotides
The nitrogenous base of a nucleotide always joins the carbon in position 1 of the corresponding pentose, through a covalent N-glycosidic bond.
In particular,
- The nitrogenous bases that derive from pyrimidine they form the N-glycosidic bond, through their nitrogen in position 1;
- While the nitrogenous bases that derive from purine they form the N-glycosidic bond, through their nitrogen in position 9.
In the chemical structure of nucleotides, the pentose represents the central element, to which the nitrogenous base and the phosphate group bind.
The chemical bond that joins the phosphate group to the pentose is of the phosphodiester type and involves an oxygen of the phosphate group and the carbon in position 5 of the pentose.
WHEN DO NITROGEN BASES FORM A NUCLEOSIDE?
The combination of a nitrogenous base and a pentose forms an organic molecule which takes the name of nucleoside.
Hence, it is the addition of the phosphate group that changes the nucleosides into nucleotides.
Moreover, according to a particular definition of nucleotides, these organic compounds would be "nucleosides that have one or more phosphate groups linked to carbon 5 of the constituent pentose".
Organization in the DNA
DNA, or deoxyribonucleic acid, is a large biological molecule made up of two very long strands of nucleotides (or polynucleotide strands).
These polynucleotide filaments have some characteristics, which deserve a particular mention because they also closely affect the nitrogenous bases:
- They are united with each other.
- They are oriented in opposite directions ("antiparallel filaments").
- They wrap around each other, as if they were two spirals.
- The nucleotides that constitute them have such an arrangement, so that the nitrogenous bases are oriented towards the central axis of each spiral, while the pentoses and the phosphate groups form the external scaffolding of the latter.
The singular arrangement of the nucleotides causes each nitrogenous base of one of the two polynucleotide filaments to join, through hydrogen bonds, to a nitrogenous base present on the other filament. This union, therefore, creates a pairing of bases, pairing that biological and geneticists call it pairing or base pair.
Poc "indeed it has been affirmed that the two filaments are joined together: to determine the union are the bonds existing between the various nitrogenous bases of the two polynucleotide filaments.
CONCEPT OF COMPLEMENTARITY BETWEEN NITROGEN BASES
By studying the structure of DNA, the researchers found that the pairing between nitrogenous bases is highly specific. In fact, they noticed that adenine only binds to thymine, while cytosine only binds to guanine.
In light of this discovery, they coined the term “complementarity between nitrogenous bases”, to indicate the univocal bond between adenine with thymine and cytosine with guanine.
The identification of complementary pairing between nitrogenous bases represented the keystone, to explain the physical dimensions of the DNA and the particular stability enjoyed by the two polynucleotide strands.
The American biologist James Watson and the English biologist Francis Crick, in 1953, made a decisive contribution to the discovery of the structure of DNA (from the "spiral coiling of the two polynucleotide strands" to the pairing between complementary nitrogenous bases).
With the formulation of the so-called "double helix model", Watson and Crick had an "incredible intuition, which represented an epochal turning point in the field of molecular biology and genetics.
In fact, the discovery of the exact structure of DNA made it possible to study and understand the biological processes involving deoxyribonucleic acid: from how RNA replicates or forms to how it generates proteins.

THE BONDS THAT BIND THE PAIRS OF NITROGEN BASES TOGETHER
To unite two nitrogenous bases in a DNA molecule, forming complementary pairs, are a series of chemical bonds, known as hydrogen bonds.
Adenine and thymine interact with each other by means of two hydrogen bonds, while guanine and cytosine by means of three hydrogen bonds.
HOW MANY PAIRS OF NITROGEN BASES DOES A HUMAN DNA MOLECULE CONTAIN?
A generic human DNA molecule contains about 3.3 billion nitrogenous base pairs, which is about 3.3 billion nucleotides per strand.

Figure: chemical interaction between adenine and thymine and between guanine and cytosine. The reader can note the position and number of hydrogen bonds that hold together the nitrogenous bases of two polynucleotide strands.
Organization in the RNA
Unlike DNA, RNA, or ribonucleic acid, is a nucleic acid that is usually composed of a single strand of nucleotides.
Therefore, the nitrogenous bases that constitute it are "unpaired".
However, it should be pointed out that the lack of a complementary nitrogenous base strand does not exclude the possibility that the nitrogenous bases of RNA can pair up like those of DNA.
In other words, the nitrogenous bases of a single RNA strand can pair, according to the laws of complementarity between nitrogenous bases, just like the nitrogenous bases of DNA.
The complementary pairing between nitrogenous bases of two distinct RNA molecules is the basis of the important process of protein synthesis (or protein synthesis).
THE URACILE REPLACES TIMINA
In the "RNA," uracil replaces the thymine of the DNA not only in the structure, but also in the complementary pairing: in fact, it is the nitrogenous base that binds specifically to the adenine, when two distinct molecules of RNA appear for functional reasons.
Biological role
The expression of genes depends on the sequence of nitrogenous bases joined to the DNA nucleotides. Genes are more or less long segments of DNA (therefore segments of nucleotides), which contain the information indispensable for the synthesis of proteins. Made up of amino acids, proteins they are biological macromolecules, which play a fundamental role in regulating the cellular mechanisms of an organism.
The nitrogenous base sequence of a given gene specifies the amino acid sequence of the related protein.











.jpg)











