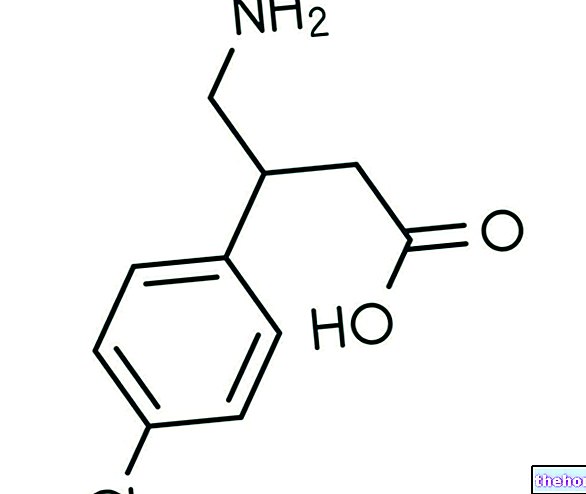
Active ingredients: Levothyroxine (Levothyroxine sodium)
Syntroxine 13 mcg Soft Capsules
Syntroxine 25 mcg Soft Capsules
Syntroxine 50 mcg Soft Capsules
Syntroxine 75 mcg Soft Capsules
Syntroxine 88 mcg Soft Capsules
Syntroxine 100 mcg Soft Capsules
Syntroxine 112 mcg Soft Capsules
Syntroxine 125 mcg Soft Capsules
Syntroxine 137 mcg Soft Capsules
Syntroxine 150 mcg Soft Capsules
Syntroxine 175 mcg Soft Capsules
Syntroxine 200 mcg Soft Capsules
Source Package Leaflet: AIFA (Italian Medicines Agency). Content published in January 2016. The information present may not be up-to-date.
To have access to the most up-to-date version, it is advisable to access the AIFA (Italian Medicines Agency) website. Disclaimer and useful information.
01.0 NAME OF THE MEDICINAL PRODUCT - 02.0 QUALITATIVE AND QUANTITATIVE COMPOSITION - 03.0 PHARMACEUTICAL FORM - 04.0 CLINICAL PARTICULARS - 04.1 Therapeutic indications - 04.2 Posology and method of administration - 04.3 Contraindications - 04.4 Special warnings and appropriate precautions for use - 04.5 Interactions with other medicinal products and other forms of interaction - 04.6 Pregnancy and lactation - 04.7 Effects on the ability to drive and use machines - 04.8 Undesirable effects - 04.9 Overdose - 05.0 PHARMACOLOGICAL PROPERTIES - 05.1 "Pharmacodynamic properties - 05.2 Pharmacokinetic properties" - 05.3 Preclinical safety data - 06.0 PHARMACEUTICAL PARTICULARS - 06.1 Excipients - 06.2 Incompatibility "- 06.3 Shelf life" - 06.4 Special precautions for storage - 06.5 Nature of the primary packaging and contents of the package - 06.6 Instructions for use and handling - 07.0 AUTHORIZATION HOLDER ALL "PLACING ON THE MARKET - 08.0 MARKETING AUTHORIZATION NUMBER - 09.0 DATE OF FIRST AUTHORIZATION OR RENEWAL OF THE AUTHORIZATION - 10.0 DATE OF REVISION OF THE TEXT - 11.0 FOR RADIO DRUGS, COMPLETE DATA ON INTERNAL RADIATION DOSIMETRY - 12.0 INSTRUCTIONS FOR RADIOPHONES ON EXTEMPORARY PREPARATION AND QUALITY CONTROL -
01.0 NAME OF THE MEDICINAL PRODUCT -
SYNTROXINE SOFT CAPSULES
02.0 QUALITATIVE AND QUANTITATIVE COMPOSITION -
1 soft capsule of Syntroxine 13 mcg contains 13 mcg of sodium levothyroxine.
1 soft capsule of Syntroxine 25 mcg contains 25 mcg of sodium levothyroxine.
1 soft capsule of Syntroxine 50 mcg contains 50 mcg of sodium levothyroxine.
1 soft capsule of Syntroxine 75 mcg contains 75 mcg of sodium levothyroxine.
1 soft capsule of Syntroxine 88 mcg contains 88 mcg of sodium levothyroxine.
1 soft capsule of Syntroxine 100 mcg contains 100 mcg of sodium levothyroxine.
1 soft capsule of Syntroxine 112 mcg contains 112 mcg of levothyroxine sodium.
1 soft capsule of Syntroxine 125 mcg contains 125 mcg of sodium levothyroxine.
1 soft capsule of Syntroxine 137 mcg contains 137 mcg of levothyroxine sodium.
1 soft capsule of Syntroxine 150 mcg contains 150 mcg of sodium levothyroxine.
1 soft capsule of Syntroxine 175 mcg contains 175 mcg of sodium levothyroxine.
1 soft capsule of Syntroxine 200 mcg contains 200 mcg of sodium levothyroxine.
For the full list of excipients, see section 6.1.
03.0 PHARMACEUTICAL FORM -
Soft capsule
Soft, oval, round, amber colored capsules.
Each capsule is marked with a mark (letter) identifying the dosage.
The soft capsules of Syntroxine 13 mcg are identified by the letter "A".
The soft capsules of Syntroxine 25 mcg are identified by the letter "E".
The soft capsules of Syntroxine 50 mcg are identified by the letter "G".
The soft capsules of Syntroxine 75 mcg are identified by the letter "H".
The soft capsules of Syntroxine 88 mcg are identified by the letter "J".
The soft capsules of Syntroxine 100 mcg are identified by the letter "K".
The soft capsules of Syntroxine 112 mcg are identified by the letter "M".
The soft capsules of Syntroxine 125 mcg are identified by the letter "N".
The soft capsules of Syntroxine 137 mcg are identified by the letter "P".
The soft capsules of Syntroxine 150 mcg are identified by the letter "S".
The soft capsules of Syntroxine 175 mcg are identified by the letter "U".
The soft capsules of Syntroxine 200 mcg are identified by the letter "Y".
04.0 CLINICAL INFORMATION -
04.1 Therapeutic indications -
Syntroxine 25-200 mcg soft capsules
- Treatment of benign euthyroid goiter
- Prophylaxis of recurrent goiter after resection of euthyroid goiter, depending on the postoperative hormonal status
- Thyroid hormone replacement therapy in hypothyroidism
- Suppressive therapy in case of malignant thyroid cancer
- supportive therapy in the thyrostatic treatment of hyperthyroidism
- thyroid suppression test
Syntroxine 13 mcg soft capsules
- In children, as an initial dose of thyroid hormone replacement therapy in cases of hypothyroidism
- In elderly patients, coronary artery patients and those with severe or chronic hypothyroidism as a low starting dose which subsequently should be increased slowly and at prolonged intervals (e.g. a gradual dose increase of 13 micrograms every 14 days) with monitoring frequent thyroid hormone values
- In all those patients in which it is necessary to gradually increase the dose of levothyroxine.
04.2 Posology and method of administration -
To ensure that patients can be treated according to their individual needs, soft capsules are available with dosages ranging from 13 to 200 mcg of levothyroxine sodium, ideally making it possible to take only one soft capsule per day. .
The posology instructions should be interpreted as guidelines.
The individual daily dose should be determined by laboratory diagnostic tests and clinical investigations.
Considering that some patients on therapy show elevated concentrations of T4 and fT4, the measurement of the basal serum concentration of thyroid stimulating hormone (TSH) is a more reliable parameter for determining further therapeutic procedures.
With the exception of neonates, in whom rapid (hormone) replacement therapy is indicated, thyroid hormone treatment should be initiated with a low dose which should be increased continuously every 2 to 4 weeks until the maintenance dose is complete.
In elderly patients, in those with coronary artery disease and in patients in whom hypothyroidism is severe or chronic, thyroid hormone treatment should be initiated with particular caution. A low starting dose should be chosen (eg 13 micrograms / day). ) which should be increased slowly and at prolonged intervals (e.g. a gradual dose increase of 13 micrograms every 14 days), with frequent monitoring of thyroid hormone values. In this case, administration of a dose that is lower than that required for complete replacement and which is not sufficient to bring the TSH value completely back to normal.
Experience shows that lower doses are sufficient even in cases of low body weight and voluminous adenomatous goiter.
Dosage: see table.
The total daily dose can be administered as a single dose.
Ingestion: The total daily dose should be swallowed whole with liquid (eg half a glass of water) in the morning, on an empty stomach, at least half an hour before breakfast.
Duration of treatment: usually ad vitam treatment in cases of hypothyroidism, strumectomy or thyroidectomy for malignant thyroid tumor, and in the prophylaxis of relapses after strumectomy of a euthyroid goiter. In the supportive therapy of hyperthyroidism for the duration of treatment with thyrostatic drugs.
In benign euthyroid goiter, treatment ranges from a period of 6 months to 2 years. If drug treatment is insufficient during this period, surgery or radioiodine therapy for goiter should be considered.
Children
Syntroxine can be given to children, but only if they are able to swallow a whole capsule. Syntroxine is contraindicated in children under 6 years of age.
For the recommended dose for children, see the table.
04.3 Contraindications -
Intolerance to the active substance or to any of the excipients contained in Syntroxine.
Untreated adrenocortical insufficiency, untreated hypopituitarism and untreated hyperthyroidism.
Syntroxine treatment should not be initiated in acute myocardial infarction, acute myocarditis or acute pancarditis.
Combination therapy of levothyroxine and thyroid drugs in hyperthyroidism is not indicated during pregnancy (see section 4.6).
Syntroxine is also contraindicated in individuals unable to swallow a whole soft capsule.
04.4 Special warnings and appropriate precautions for use -
Before starting thyroid hormone therapy or a thyroid suppression test, the following medical disorders or conditions should be ruled out or treated: coronary insufficiency, angina pectoris, atherosclerosis, hypertension, hypopituitarism and adrenocortical insufficiency. Similarly, autonomy of the thyroid gland must be excluded or treated before starting thyroid hormone therapy.
In patients with coronary insufficiency, heart failure or tachycardia arrhythmia, it is essential to avoid the induction of even mild pharmacological hyperthyroidism. In these cases, it is necessary to frequently monitor thyroid hormone parameters.
In secondary hypothyroidism, the cause must be established before replacement therapy is undertaken. If compensated adrenocortical insufficiency is diagnosed, appropriate replacement therapy should be undertaken if necessary.
If thyroid autonomy is suspected, a TRH test or suppression scintigraphy should be performed.
Close monitoring of thyroid function is required during levothyroxine therapy in postmenopausal hypothyroid women who are at increased risk of osteoporosis to avoid blood levels of levothyroxine above physiological levels.
Levothyroxine should not be administered in the presence of a hyperthyroid metabolic state, except as supportive therapy in the thyroidostatic treatment of hyperthyroidism.
Thyroid hormones are not suitable for weight loss. In euthyroid patients, doses that fall within the range of daily hormone requirements are not effective for weight reduction. Doses higher than physiological ones can generate serious or life-threatening side effects (see section 4.9).
If a patient on established levothyroxine therapy switches to another drug, it is recommended to adjust the dose based on the patient's clinical response and laboratory values.
For diabetic patients and on anticoagulant therapy see section 4.5.
04.5 Interactions with other medicinal products and other forms of interaction -
Antidiabetics:
Levothyroxine may reduce the effect of antidiabetic drugs. Therefore the blood sugar concentration should be monitored regularly at the start of thyroid hormone therapy and if necessary the dosage of the antidiabetic drug should be adjusted.
Coumarin derivatives:
The effect of treatment with anticoagulant could be amplified, since levothyroxine displaces anticoagulants from binding to plasma proteins. Therefore, at the beginning of treatment with thyroid hormones, the coagulation parameters must be regularly monitored and the dosage of the anticoagulant it must be adjusted if necessary.
Cholestyramine, colestipol:
The intake of ion exchange resins, such as cholestyramine and colestipol, inhibits the absorption of levothyroxine. Levothyroxine should therefore be taken 4 - 5 hours prior to the administration of these medicinal products.
Preparations containing aluminum or iron, calcium carbonate:
The literature reports that aluminum-containing preparations (antacids, sucralfate) have the ability to reduce the efficacy of levothyroxine. Therefore, levothyroxine should be taken at least two hours before any preparation containing aluminum.
The same applies to preparations containing iron or calcium carbonate.
Salicylates, dicumarol, furosemide, clofibrate, phenytoin:
Levothyroxine can be displaced from its plasma protein binding by salicylates, dicumarol, high-dose furosemide (250 mg), clofibrate, phenytoin and other substances, resulting in an increase in the fT4 fraction.
Propylthiouracil, glucocorticoids, beta-sympatholytic agents, amiodarone and iodine-containing contrast media:
These substances inhibit the peripheral conversion of T4 to T3.
Amiodarone: has a high iodine content that can induce hyperthyroidism or hypothyroidism. Particular caution is required in cases of nodular goiter, with possible undiagnosed thyroid autonomy.
Sertraline, chloroquine / proguanil:
These substances reduce the effectiveness of levothyroxine and lead to an increase in TSH.
Drugs with enzyme-inducing effect:
Drugs with hepatic enzyme inducing effects, such as barbiturates, may increase the hepatic clearance of levothyroxine.
Estrogen:
In women taking estrogen-containing contraceptives, or in postmenopausal women on hormone replacement therapy, the need for levothyroxine may increase.
Protease inhibitors:
Levothyroxine has been reported to lose therapeutic efficacy when administered concomitantly with lopinavir / ritonavir. Therefore, careful monitoring of thyroid function is necessary in patients taking levothyroxine and protease inhibitors at the same time.
Sevelamer:
It has been reported that sevelamer may increase TSH levels in patients administered concomitantly with levothyroxine. Therefore, careful monitoring of TSH levels is advised in patients treated with both drugs.
Orlistat:
Hypothyroidism and / or reduced control of hypothyroidism may occur when orlistat and levothyroxine are taken simultaneously. This may be due to reduced absorption of iodine salts and / or levothyroxine.
Patients taking levothyroxine should consult their doctor before starting treatment with drugs containing orlistat (eg Alli), as it may be necessary to take orlistat and levothyroxine at different times and adjust the levothyroxine dosage.
Soy-based products:
Products containing soya may reduce the intestinal absorption of Syntroxine. In particular, at the start of therapy or after a diet containing soya, the dosage of Syntroxine may need to be adjusted.
04.6 Pregnancy and breastfeeding -
Pregnancy
Experience in humans has shown that there is no evidence of drug induced teratogenicity or toxicity to the fetus / neonatal during pregnancy at recommended therapeutic dosages.
Neonatal development depends on maternal thyroid function. Thyroxine is necessary for the brain development of the newborn. It follows that continuous treatment with thyroid hormones must be maintained, especially during pregnancy. An increase in dosage may be necessary during pregnancy.
Feeding time
Levothyroxine is secreted into breast milk during lactation; however, the concentrations reached at the recommended dosage regimen are not sufficient to cause the development of hyperthyroidism or the suppression of TSH secretion in the newborn. Levothyroxine can be used during lactation.
Use as support therapy with thyrostatics
Levothyroxine should not be administered in combination with thyreostatic drugs for the treatment of hyperthyroidism during pregnancy and lactation. Levothyroxine may require a higher dose of thyreostatic drug.
Since thyrostatic drugs cross the placenta more easily than levothyroxine, a combination therapy could induce hypothyroidism in the fetus. Therefore only thyreostatics should be used in the treatment of hyperthyroidism during pregnancy.
04.7 Effects on ability to drive and use machines -
No studies on the ability to drive and use machines have been performed. However, given the fact that levothyroxine is identical to natural thyroid hormone, Syntroxine is not expected to affect the ability to drive and use machines.
04.8 Undesirable effects -
With appropriate use and monitoring of clinical reports and laboratory diagnostic values, no undesirable effects are expected during treatment with Syntroxine. In isolated cases, the dose may not be tolerated, or the patient may have taken an overdose. In these cases, particularly when the dose has been increased too rapidly at the start of treatment, symptoms similar to those seen in hyperthyroidism may occur, such as tachycardia, palpitations, cardiac arrhythmias, angina pectoris, headache, weakness and muscle cramps. flushing, fever, vomiting, menstrual disturbances, pseudotumor cerebri, tremor, restlessness, insomnia, hyperhidrosis, weight loss and diarrhea.
In these cases, the daily dose should be reduced or the drug suspended for several days. As soon as the adverse effect subsides, it is possible to resume treatment, with a careful dosage regimen.
In case of hypersensitivity to any of the excipients of Syntroxine, skin and respiratory tract reactions may occur.
Reporting of suspected adverse reactions
Reporting of suspected adverse reactions occurring after authorization of the medicinal product is important as it allows continuous monitoring of the benefit / risk balance of the medicinal product. Healthcare professionals are asked to report any suspected adverse reactions via the national reporting system. "address www.agenziafarmaco.gov.it/it/responsabili.
04.9 Overdose -
A high T3 value is a more reliable overdose index than high T4 or fT4 values.
In the event of an overdose, symptoms suggestive of a marked increase in metabolic activity appear (see section 4.8). Depending on the extent of the overdose, it is recommended that the patient stop taking the soft capsules and be monitored.
Symptoms can manifest themselves in the form of marked beta-adrenergic effects, such as tachycardia, anxiety, agitation and hyperkinesis. Symptoms can be reduced by beta blockers. For excessive dosages, plasmapheresis may be useful.
In case of overdose in humans (with suicidal intent) doses of 10 mg of levothyroxine were tolerated without complications.
Cases of cardiac arrest have been reported in patients who have misused levothyroxine over many years.
05.0 PHARMACOLOGICAL PROPERTIES -
05.1 "Pharmacodynamic properties -
Pharmacotherapeutic group: thyroid hormones
ATC code: H03A A01
The synthetic levothyroxine contained in Syntroxine has the same action as the natural thyroid hormone produced mainly by the thyroid gland. It is transformed into T3 in the peripheral organs and, like the natural hormone, exerts its characteristic effects at the level of the T3 receptors. The body is unable to distinguish between endogenous and exogenous levothyroxine.
05.2 "Pharmacokinetic properties -
Levothyroxine administered orally is almost exclusively absorbed in the upper tract of the small intestine. Depending on the nature of the pharmaceutical formulation, up to a maximum of 80% is absorbed. The Tmax is between 1 and 6 hours.
Once oral therapy is started, the effects appear after 3 - 5 days. Levothyroxine is strongly bound to plasma proteins by 99.97%. Since no covalent bonds are formed, there is a continuous and very rapid exchange between the fraction of the hormone bound to the proteins and the fraction of free hormone.
Due to its strong protein binding, levothyroxine cannot be removed from the body by hemodialysis or hemoperfusion.
On average, the half-life of levothyroxine is about 7 days. In hyperthyroidism, it is shorter (3 - 4 days), while in hypothyroidism it is longer (about 9 - 10 days). The volume of distribution is between 10 and 12 l. One third of the levothyroxine produced externally to the thyroid is present in the liver, and can be rapidly exchanged for serum levothyroxine. Thyroid hormones are predominantly metabolised in the liver, kidney, brain and muscle. The metabolites are excreted in the urine and faeces Metabolic clearance is approximately 1.2 L of plasma / day.
05.3 Preclinical safety data -
Acute toxicity:
The acute toxicity of levothyroxine is very low.
Chronic toxicity:
Chronic toxicity studies have been conducted in numerous animal species (rat, dog). At high doses, signs of liver disease, an "increased incidence of spontaneous nephrosis and changes in organ weight were observed in rats."
Reproductive toxicity:
Reproductive toxicity studies in animals have not been performed.
Mutagenicity:
No data are available on the mutagenic potential of levothyroxine. However, to date, no suspected cases or evidence have been reported to suggest the involvement of thyroid hormones in damaging the offspring by altering the genome.
Carcinogenicity:
Chronic toxicity studies with levothyroxine have not been conducted in animals.
06.0 PHARMACEUTICAL INFORMATION -
06.1 Excipients -
Jelly
Glycerol
Purified water
06.2 Incompatibility "-
Not relevant.
06.3 Period of validity "-
2 years.
06.4 Special precautions for storage -
Do not store above 25 ° C.
06.5 Nature of the immediate packaging and contents of the package -
PVC-polychlorotrifluoroethylene (PCTFE) / aluminum blisters
Packaging: 30, 50 and 100 soft capsules
Not all pack sizes may be marketed.
06.6 Instructions for use and handling -
No special instructions.
07.0 HOLDER OF THE "MARKETING AUTHORIZATION" -
Bracco S.p.A. - via E. Folli, 50 - 20134 Milan
Licensed by IBSA
08.0 MARKETING AUTHORIZATION NUMBER -
AIC 041528011 "13 mcg soft capsules" 30 capsules in Pvc-Pctfe / Al blister
AIC 041528023 "13 mcg soft capsules" 50 capsules in Pvc-Pctfe / Al blister
AIC 041528035 "13 mcg soft capsules" 100 capsules in Pvc-Pctfe / Al blister
AIC 041528047 "25 mcg soft capsules" 30 capsules in Pvc-Pctfe / Al blister
AIC 041528050 "25 mcg soft capsules" 50 capsules in Pvc-Pctfe / Al blister
AIC 041528062 "25 mcg soft capsules" 100 capsules in Pvc-Pctfe / Al blister
AIC 041528074 "50 mcg soft capsules" 30 capsules in Pvc-Pctfe / Al blister
AIC 041528086 "50 mcg soft capsules" 50 capsules in Pvc-Pctfe / Al blister
AIC 041528098 "50 mcg soft capsules" 100 capsules in Pvc-Pctfe / Al blister
AIC 041528100 "75 mcg soft capsules" 30 capsules in Pvc-Pctfe / Al blister
AIC 041528112 "75 mcg soft capsules" 50 capsules in Pvc-Pctfe / Al blister
AIC 041528124 "75 mcg soft capsules" 100 capsules in Pvc-Pctfe / Al blister
AIC 041528136 "88 mcg soft capsules" 30 capsules in Pvc-Pctfe / Al blister
AIC 041528148 "88 mcg soft capsules" 50 capsules in Pvc-Pctfe / Al blister
AIC 041528151 "88 mcg soft capsules" 100 capsules in Pvc-Pctfe / Al blister
AIC 041528163 "100 mcg soft capsules" 30 capsules in Pvc-Pctfe / Al blister
AIC 041528175 "100 mcg soft capsules" 50 capsules in Pvc-Pctfe / Al blister
AIC 041528187 "100 mcg soft capsules" 100 capsules in Pvc-Pctfe / Al blister
AIC 041528199 "112 mcg soft capsules" 30 Capsules In Blister Pvc-Pctfe / Al
AIC 041528201 "112 mcg soft capsules" 50 Capsules In Blister Pvc-Pctfe / Al
AIC 041528213 "112 mcg soft capsules" 100 Capsules In Blister Pvc-Pctfe / Al
AIC 041528225 "125 mcg soft capsules" 30 capsules in Pvc-Pctfe / Al blister
AIC 041528237 "125 mcg soft capsules" 50 capsules in Pvc-Pctfe / Al blister
AIC 041528249 "125 mcg soft capsules" 100 capsules in Pvc-Pctfe / Al blister
AIC 041528252 "137 mcg soft capsules" 30 capsules in Pvc-Pctfe / Al blister
AIC 041528264 "137 mcg soft capsules" 50 capsules in Pvc-Pctfe / Al blister
AIC 041528276 "137 mcg soft capsules" 100 capsules in Pvc-Pctfe / Al blister
AIC 041528288 "150 mcg soft capsules" 30 capsules in Pvc-Pctfe / Al blister
AIC 041528290 "150 mcg soft capsules" 50 capsules in Pvc-Pctfe / Al blister
AIC 041528302 "150 mcg soft capsules" 100 capsules in Pvc-Pctfe / Al blister
AIC 041528314 "175 mcg soft capsules" 30 capsules in Pvc-Pctfe / Al blister
AIC 041528326 "175 mcg soft capsules" 50 capsules in Pvc-Pctfe / Al blister
AIC 041528338 "175 mcg soft capsules" 100 capsules in Pvc-Pctfe / Al blister
AIC 041528340 "200 mcg soft capsules" 30 capsules in Pvc-Pctfe / Al blister
AIC 041528353 "200 mcg soft capsules" 50 capsules in Pvc-Pctfe / Al blister
AIC 041528365 "200 mcg soft capsules" 100 capsules in Pvc-Pctfe / Al blister
09.0 DATE OF FIRST AUTHORIZATION OR RENEWAL OF THE AUTHORIZATION -
03/09/2012
10.0 DATE OF REVISION OF THE TEXT -
October 2016