Introduction
Platelets or thrombocytes are the smallest figured elements of the blood, with a discoid shape and a diameter between 2 and 3 µm. Unlike white blood cells (or leukocytes) and red blood cells (or erythrocytes), platelets are not actual cells, but cytoplasmic fragments of megakaryocytes located in the red marrow. These, in turn, derive from precursors called megakaryoblasts and appear as large multinucleated cells (diameter from 20 to 15 nm), which after various stages of maturation undergo phenomena of cytoplasmic fragmentation, originating from 2000 to 4000 platelets. Consequently, thrombocytes are devoid of nucleus (like red blood cells) and of structures such as the endoplasmic reticulum and the Golgi apparatus; however, they are delimited by a membrane, which makes each platelet independent from the others, and possess granules, various organelles cytoplasmic and RNA.
As anticipated, the dimensions of the platelets are particularly small; despite this their internal structure is extremely complex, since they intervene in a biological process of primary importance called haemostasis [haima, blood + stasis block]. In synergy with the coagulation enzymes, the platelets allow the passage of blood from the fluid to the solid state, forming a kind of plug (or thrombus) that obstructs the injured points of the vessels.
Normal values in the blood
150,000 to 400,000 platelets are normally present in one milliliter of blood. Their average life is 10 days (compared to 120 for red blood cells), at the end of which they are phagocytized or destroyed by macrophages, especially in the liver and spleen (in the latter there is approximately one third of the total platelet mass). Every day from 30,000 to 40,000 platelets per mm3 are produced; if necessary, this synthesis can increase 8 times.
Platelet structure
The structure of platelets is extremely complex, so that they are activated only in response to precise and well-determined stimuli; if this were not the case, platelet aggregation in circumstances that are not strictly necessary, or a defect at the time of need, would have very serious consequences for the organism (pathological thrombogenesis and haemorrhages).
Since incorrect blood clotting plays a primary role in the genesis of strokes and heart attacks, the biological mechanisms that control it are still the subject of numerous studies.
Platelets are always present in the circulation, but are activated only when there is damage to the walls of the circulatory system.
The structure of platelets, as well as their shape and volume, change profoundly in relation to the degree and stage of activity. In the inactive form, platelets consist of a paler part (hyalomer) and a more refractive central part (chromomer), rich in granules containing coagulation proteins and cytokines. The cell membrane is rich in protein molecules and glycoproteins, which act as receptors by regulating the interaction of the platelet with the surrounding environment (adhesion and aggregation).
Coagulation and platelets
Platelets are just some of the many actors involved in the clotting process. Following the injury of a blood vessel, the release of some chemical substances by the endothelial cells, and the exposure of the collagen of the damaged wall, determine the activation of platelets (the endothelium is a particular lining tissue of the internal surface of blood vessels, which under normal conditions separates the collagen matrix fibers from the blood preventing platelet adhesion).
Platelets quickly adhere to collagen exposed in the damaged wall (platelet adhesion) and are activated by releasing particular substances (called cytokines) into the lesion area. These factors promote the activation and association of other platelets, which aggregate to form a fragile plug, the so-called white thrombus; moreover, they help to reinforce the local vasoconstriction previously triggered by some paracrine substances, released by the injured endothelium with the aim of decreasing blood flow and pressure. Both reactions are mediated by the release of substances contained within some platelet granules, such as serotonin, calcium, ADP and platelet activating factor (PAF). The latter triggers a signaling pathway that converts the phospholipids of the platelet membrane into thromboxane A2, which has a vasoconstrictive action and promotes platelet aggregation.
Platelets are extremely fragile: a few seconds after the injury of a vessel they aggregate and break, releasing the contents of their granules into the surrounding blood and favoring the formation of a clot.
The "aggregation of thrombocytes must" obviously be limited to prevent the platelet plug from extending into areas not affected by endothelial damage; platelet adhesion to healthy vessel walls is thus limited by the release of NO and prostacyclin (an eicosanoid).
The primary platelet plug is consolidated in the next phase, in which a series of reactions rapidly follow one another

While on the one hand the prostacyclin released by the cells of the healthy endothelium inhibits platelet adhesion, on the other hand our body synthesizes anticoagulants - such as heparin, antithrombin III and protein C - to block and regulate some of the reactions involved. in the coagulation cascade, which must necessarily be confined to the injured area.
Vascular phase → reduction of the vascular lumen
Contraction of the vascular musculature
Peripheral vasoconstriction
Platelet phase → formation of the platelet plug
Membership
Shape change
Degranulation
Aggregation
Coagulation phase → fibrin clot formation:
Cascade of enzymatic reactions
Fibrinolytic phase → dissolution of the clot:
Activation of the fibrinolytic system
Platelets play an essential role in "arresting" bleeding, but they do not directly intervene in the repair of the damaged vessel, which is instead due to processes of cell growth and division (fibroblasts and vascular smooth muscle cells).Once the leak has been repaired the clot dissolves slowly and retracts by the action of the enzyme plasmin trapped inside the clot.
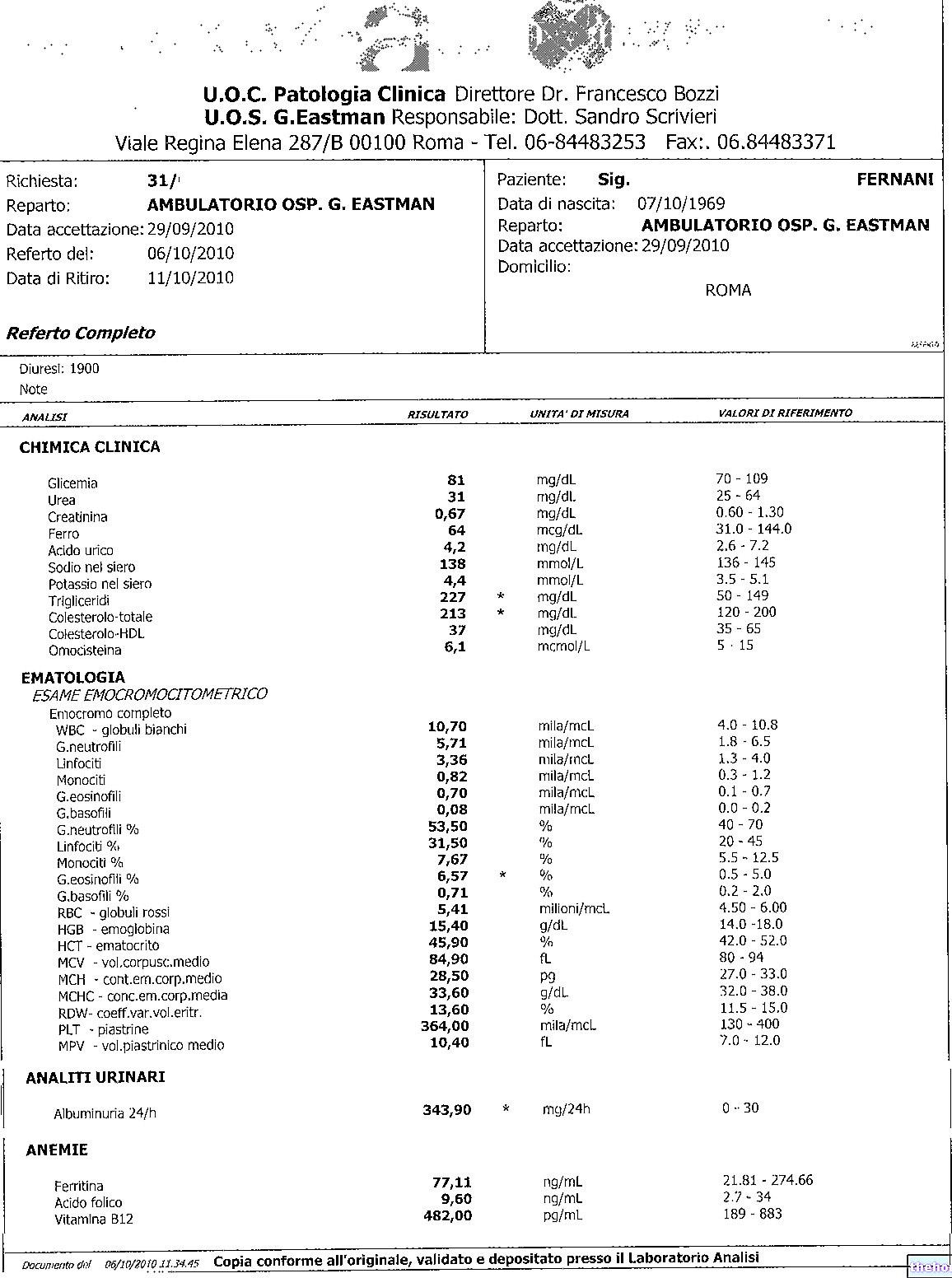
Pistrine and blood tests
- PLT: platelet count, number of platelets per blood volume
- MPV: mean platelet volume
- PDW: distribution width of platelet volumes (index of platelet anisocytosis)
- PCT: or platelet hematocrit, volume of blood occupied by the pistrins