See also: urine pH; Vaginal pH.
The pH of blood, and of any other fluid, reflects the concentration of hydrogen ions (H +) dissolved in it. A pH value of 7 is neutral; lower and higher values are respectively acidic and basic.

By increasing the ventilation, ie the respiratory rate and / or the depth of breath, the body increases the amount of carbon dioxide excreted, raising the pH of the blood. Conversely, in the opposite case (hypoventilation occurs as a result of blood alkalosis).
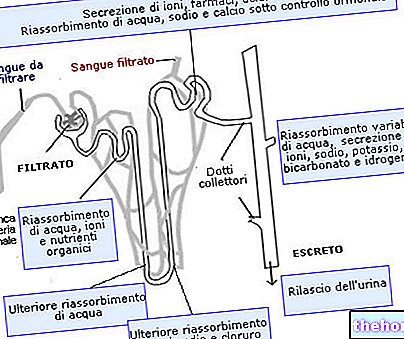
In the kidney there is another very important compensatory mechanism of the blood pH, even if it is much slower to set in motion. The cells of the nephrons can in fact respond to acidosis by reabsorbing greater quantities of bicarbonates, secreting greater quantities of hydrogen ions, reabsorbing more buffers (HCO3-) and promoting the genesis of ammonia (which has the ability to react with free H + ions, forming the ion ammonium: NH3 + H + <→ NH4 +).
- HOMEOSTASIS OF BLOOD pH DEPENDS ON BUFFER SYSTEMS, LUNG AND KIDNEY
Acidosis and alkalosis can have a "respiratory or metabolic origin. In the first case they are due to an excess or defect of carbon dioxide, while in the second they are associated with a deficit or surplus of non-volatile metabolic substances, therefore not eliminable with the breath.
Please note: carbon dioxide itself is not acidic, because it does not contain any hydrogen atoms. However, in the blood environment it combines with water to form carbonic acid, which dissociates into H + and HCO3-; by law of mass action, if the concentration of carbon dioxide increases (see figure in red), the blood environment becomes acidified. In the opposite condition (green color), the situation is reversed.



- Lactic acidosis (due to hypoxia or hypoperfusion, common in physical exercise);
- Ketoacidosis (massive production of ketone bodies typical of diabetes), ketosis (important production of ketone bodies typical of particularly prolonged fasting or severe malnutrition);
- Kidney failure;
- Poisoning;
- Severe diarrhea;
- Hypoventilation (caused by drugs, drugs, particularly serious diseases, COPD and in a mild form also typical of severely obese subjects).
- He retched;
- Excessive consumption of alkalizing agents or drugs (such as baking soda)
- Hyperventilation (including that induced by drugs or by artificial respiration or drugs).











.jpg)











