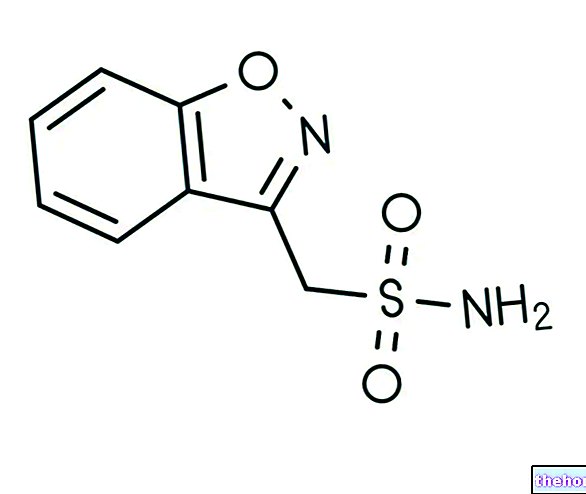
However, due to its liver toxicity, nimesulide is used as a second-line drug and - for the same reason - has been withdrawn from the market in some European countries.
In Italy it is still marketed, but the dispensing of most of the medicines that contain it can only take place upon presentation of a non-repeatable medical prescription (RNR); however, since these are class A drugs, their cost can be reimbursed by the National Healthcare System (SSN), wholly or partially, depending on the case (it may be necessary to pay a ticket).
Nimesulide is available in pharmaceutical formulations suitable for oral (granules or powder for oral suspension, tablets, orodispersible tablets) and cutaneous (skin gel) administration.
The skin gel based on nimesulide is classified as an SOP drug (Without Obligation of Medical Prescription); in addition, nimesulide-based mouthwashes are also available on the market, dispensable upon presentation of a repeatable medical prescription (RR). The cost of both these types of medicines, being classified as class C drugs, is entirely borne by the citizen.