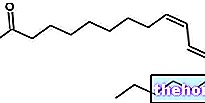
Palmitoleic acid is a monounsaturated, non-essential fatty acid of the omega 7 series. It is therefore a molecule formed by a long carbon chain (16 carbon atoms in all), which begins with a carboxylic group (COOH) and ends with a methyl group (CH3) and has in the central part a series of carbon atoms respectively coupled to two hydrogen atoms. An exception to that described is a single Carbon-Carbon pair, which - held together by a double bond - binds only one hydrogen atom per carbonaceous unit. This pair involves the seventh and eighth carbon starting from the methyl end (terminal), which explains why palmitoleic acid belongs to the omega 7 series.

Natural sources of palmitoleic acid are quite numerous, but the content is significant only in sea buckthorn oil (Hippophae rhamnoides) and in that of macadamia (Macadamia integrifolia); these oils contain, respectively, about 40 and 17% of palmitoleic acid.
As mentioned, this nutrient can be synthesized by the body from other fatty acids, in particular from palmitic (C16: 0) by the intervention of the enzyme delta nine desaturase (palmitoleic is both an omega 7 and a delta 9, given that if you start counting starting from the carboxyl end, the first carbon atom engaged in the double bond is the number 9).
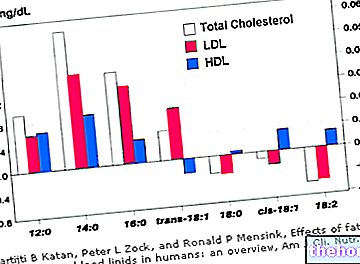
Although it belongs to the group of monounsaturated fatty acids, from the health point of view palmitoleic acid is comparable to palmitic, a saturated fatty acid with a pro-atherogenic effect:
a supplementation with palmitoleic acid increases the levels of bad cholesterol, LDL, in hypercholesterolemic patients, even when the dietary intake of cholesterol is low; this increase is comparable to that obtained through palmitic acid supplementation, but significantly higher than the induced one from the integration with oleic acid. Furthermore, compared to palmitic acid, palmitoleic caused a greater reduction in good HDL cholesterol.
From a health point of view, it is therefore inadvisable to replace traditional sources of unsaturated fatty acids (olive oil, seed oils and fish oils) with sea buckthorn or macadamia oil.
Cosmetic products containing sources of palmitoleic acid have emollient and moisturizing properties. However, this fatty acid, along with other members of the omega-7 series, has been pointed out as possibly responsible for the characteristic odor of aged skin.
Recently, "anti-fattening" properties have also been ascribed to "palmitoleic acid", due to its ability to act as a signal molecule that prevents the accumulation of dietary fats in adipose reserves (in genetically modified rats); palmitoleic acid seems to stimulate the action of insulin in the muscles and oppose hepatic steatosis.