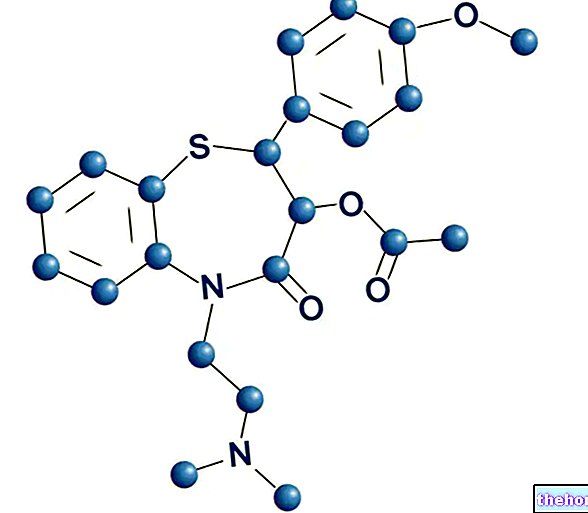
SOLARAZE ® is a sodium diclofenac-based drug
THERAPEUTIC GROUP: Other dermatological preparations

Indications SOLARAZE ® Diclofenac
SOLARAZE ® is used in the treatment of actinic keratosis.
Mechanism of action SOLARAZE ® Diclofenac
Actinic keratosis, also called solar keratosis, is a dermatological pathology characterized by the presence of patches up to one cm in size, scaly, yellowish, rough and sometimes surrounded by telangiectasias.
The pathogenesis, not yet fully known, however, sees ultraviolet radiation as the main culprits, so much so that the sites in which these lesions are observed are those chronically exposed to the sun, such as the face, scalp, cheeks, face, hands and ears.
One of the potential therapeutic applications for this precancerous pathology, although still not supported by clear and defined molecular mechanisms, involves the topical use of non-steroidal anti-inflammatory drugs and more precisely 3% diclofenac, which has proven effective in reducing the progression and incidence of complications. .
The main therapeutic mechanism of diclofenac, also in this case, is determined by the inhibitory action exerted on cyclooxygenases, and by the relative reduction of prostaglandin concentrations, involved in the pathogenesis of actinic keratosis.
Studies carried out and clinical efficacy
1 THE MOLECULAR MECHANISMS OF DICLOFENAC IN ACTINIC KERATOSIS
Br J Dermatol. 2007 May; 156 Suppl 3: 25-33.
The role of apoptosis in therapy and prophylaxis of epithelial tumors by nonsteroidal anti-inflammatory drugs (NSAIDs).
Fecker LF, Stockfleth E, Nindl I, Ulrich C, Forschner T, Eberle J.
Study that seeks to clarify the molecular mechanisms underlying the therapeutic activity of non-steroidal anti-inflammatory drugs in the treatment of actinic keratosis. In addition to the anti-inflammatory action, this active principle seems to carry out a direct apoptotic action against hyperplastic keratinocytes.
2. DICLOFENAC AND PHOTOTOXICITY "
Eur J Dermatol. 2006 Jul-Aug; 16: 385-90.
3% diclofenac in 2.5% hyaluronic acid (Solaraze) does not induce photosensitivity or phototoxicity alone or in combination with sunscreens.
Ortonne JP, Queille-Roussel C, Duteil L.
This work demonstrates how the application of diclofenac 3% in hyaluronate at the same time as that of sunscreens does not induce phototoxic or photosensitizing reactions. This data is very important given the role of UV in the pathogenesis of actinic keratosis.
3. SOLARAZE ® E contact dermatitis
Contact Dermatitis. 2004 Oct; 51: 215-6.
Contact dermatitis from 3 different allergens in Solaraze gel.
Kleyn CE, Bharati A, King CM.
Study that demonstrates the allergenic power of some excipients present in SOLARAZE. Unfortunately this reaction, characterized by the presence of contact dermatitis, significantly limits the topical use of diclofenac, reducing its therapeutic efficacy.
Method of use and dosage
SOLARAZE ®
Gel for topical use with 3% sodium diclofenac (equal to 30 mg of active ingredient for each gram of gel)
The therapeutic treatment of solar keratoses involves taking 3% diclofenac twice a day for at least 30 days, applying a sufficient quantity of gel to cover the affected area.
To facilitate absorption, it is necessary to gently massage the part, avoiding damaging the scales.
Treatment should be supervised by your doctor
SOLARAZE ® Diclofenac warnings
Treatment with SOLARAZE ® should be supervised by your doctor, so that the latter can evaluate the efficacy and safety of the therapy implemented.
The patient should avoid contact of the drug with the eyes, mucous membranes and any damaged areas, in order to minimize the systemic absorption of the active ingredient and the appearance of side effects.
It is also useful that the patient does not expose himself to the sun during the treatment.
The presence in SOLARAZE ® of excipients such as benzyl alcohol and sodium hyaluronate could increase the risk of allergic reactions especially in atopic patients.
PREGNANCY AND BREASTFEEDING
The absence of studies relating to the side effects of topical diclofenac on fetal health and known instead those associated with oral intake, extend the contraindications also to pregnancy and the subsequent period of breastfeeding.
Interactions
The reduced systemic absorption that is observed following the topical application of diclofenac, allows to dramatically limit the possible drug interactions with other active ingredients, making the use of SOLARAZE ® as safe as possible.
Contraindications SOLARAZE ® Diclofenac
The use of SOLARAZE ® is contraindicated in patients hypersensitive to the active ingredient or to one of its excipients, as well as to salicylates and any other type of non-steroidal anti-inflammatory.
Undesirable Effects - Side Effects
Topical administration of SOLARAZE ® could be associated with the appearance of local reactions such as urticaria, skin rash, conjunctivitis and contact dermatitis.
Clinically relevant side effects have only rarely been observed, for which it was necessary to discontinue therapy.
Note
SOLARAZE ® can only be sold with a medical prescription.
The information on SOLARAZE ® Diclofenac published on this page may be out of date or incomplete. For a correct use of this information, see the Disclaimer and useful information page.