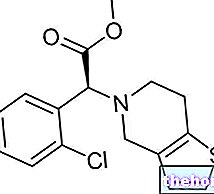
What is Selincro - Nalmefene?
Selincro is a medicine containing the active substance nalmefene, available as tablets (18 mg).
What is Selincro - Nalmefene used for?
Selincro is used to help reduce alcohol consumption in alcohol-dependent adults who consume more than 60g of alcohol per day (for men) or more than 40g of alcohol per day (for women).
The medicine should only be prescribed in conjunction with psychosocial support and only in people who do not have physical withdrawal symptoms and who do not require immediate detoxification.
As an indication, a bottle of wine (750 ml; 12% alcohol content) contains approximately 70 g of alcohol, while a bottle of beer (330 ml; 5% alcohol content) contains approximately 13 g of alcohol.
Selincro can only be obtained with a prescription.
How is Selincro used - Nalmefene?
Before starting treatment with Selincro, the patient is asked to record his alcohol consumption for a period of two weeks.
During the first visit with the physician, the patient's clinical status, alcohol dependence and alcohol consumption level (based on patient reports) should be assessed.
Thereafter, the patient will have to record his alcohol consumption for about two weeks. At the next visit (after two weeks), treatment with Selincro can be started in patients who have continued to have high alcohol consumption (over 60 g per day for men and over 40 g per day for women).
Treatment must also include psychological support, in order to help the patient reduce alcohol consumption and adhere to treatment.
The patient should take one Selincro tablet by mouth as needed, i.e. whenever there is a risk of starting drinking. Only one tablet can be taken per day, preferably 1-2 hours before the scheduled time for alcohol consumption. If the patient has started drinking alcohol without taking Selincro, one tablet should be taken as soon as possible.
The data available on the use of Selincro, taken from standard clinical studies, refer to a period of 6-12 months. Caution is recommended if Selincro is prescribed for a period longer than one year.
How does Selincro - Nalmefene work?
The active substance in Selincro, nalmefene, binds to some opiate receptors in the brain. Opiate receptors play a key role in addictions; by binding to these receptors and modifying their activity, nalmefene contributes to reducing the desire to consume alcohol in subjects accustomed to heavy consumption.
Selincro does not prevent the effects of alcohol intoxication.
How has Selincro - Nalmefene been studied?
The effects of Selincro were first tested in experimental models before being studied in humans.
Selincro has been compared with placebo (a dummy treatment) in two main studies involving 1,322 men and women with alcoholism. All patients also received psychological support, aimed at reducing alcohol consumption and facilitating patient compliance with treatment.
The main measures of effectiveness were the reduction in the number of days of high alcohol consumption and the average daily alcohol consumption six months after treatment.
What benefit has Selincro - Nalmefene shown during the studies?
Selincro has been shown to be more effective than placebo in reducing the number of high alcohol days and daily alcohol consumption.
Significant improvements, usually seen in the first four weeks of treatment, were seen in patients who already consumed more than 60 g of alcohol per day (in the case of men) or more than 40 g of alcohol per day (in women). In these patients, the number of days of high alcohol consumption in one month decreased from 23 to 10 in the first study and from 23 to 11 in the second study six months after starting treatment with Selincro. alcohol with Selincro decreased from 102 g to 44 g in the first study and from 113 g to 43 g in the second study. These data indicate a more marked improvement compared to placebo of approximately 2.7-3.7 days of high alcohol consumption per month and about 10-18 g of alcohol per day.
What is the risk associated with Selincro - Nalmefene?
The most common side effects with Selincro (seen in more than 1 in 10 patients) are nausea, dizziness, insomnia and headache. Most of these reactions were mild or moderate and of short duration.
Selincro must not be used in people who are hypersensitive (allergic) to nalmefene or any of the other ingredients. It is not indicated in patients taking opioid-containing medicines, in patients who have or have recently been opioid addictive, in people with acute opioid withdrawal symptoms, or in patients who are suspected of having recently used opioids. .
The medicine should not be used in patients with severe liver or kidney impairment or who have recently experienced acute alcohol withdrawal symptoms (including hallucinations, seizures and tremors).
Why has Selincro - Nalmefene been approved?
The CHMP noted that Selincro was shown to be effective in reducing alcohol consumption in men who reported drinking more than 60g of alcohol per day and in women who consumed more than 40g of alcohol per day. Regarding safety, the undesirable effects reported in the studies did not raise particular concern. The CHMP decided that Selincro's benefits are greater than its risks and recommended that it be given a Marketing Authorization for the medicine.
More information about Selincro - Nalmefene
On February 25, 2013 the European Commission issued a "marketing authorization" for Selincro, valid throughout the European Union.
For more information about Selincro therapy, read the package leaflet (included with the EPAR) or contact your doctor or pharmacist.
Last update of this summary: 02-2013.
The information on Selincro - Nalmefene published on this page may be out of date or incomplete. For a correct use of this information, see the Disclaimer and useful information page.