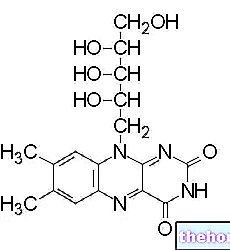
It is found in many foods (plant and animal) but in limited concentrations. There is therefore the possibility of nutritional deficiency (almost non-existent in the West), which in the most serious cases can lead to beriberi; the relative neuropathic syndrome due to alcoholism is more frequent.
Vitamin B1, in all its active forms, has numerous metabolic functions; among these above all of coenzyme catalyst - for example in the carbohydrate pathway and in the amino acid pathway.
Its requirement is about 0.4 mg for every 1000 kcal ingested. Well tolerated and rarely toxic, it can be supplemented or administered by injection; only in the latter case can it give side effects.
Let's go into detail.
For further information: Thiamine , but practically insoluble in other organic solvents. It shows stability in acidic pH, but is unstable in alkaline solutions.
In vivobeing a persistent carbene, vitamin B1 is metabolized enzymatically to catalyze benzoin condensations.
Vitamin B1 is thermolabile in cooking, but stable to freezing. It is sensitive to ultraviolet light and gamma irradiation and reacts strongly to Maillard reactions.
, some protozoa, plants and fungi. Specifically, thiazole and pyrimidine are produced separately and then combined to form thiamine monophosphate (ThMP) by the action of the thiamine-phosphate synthase enzyme (EC2.5.1.3).The biosynthetic pathways of vitamin B1 can differ between various organisms. In the E. coli and other enterobacteria, ThMP can be phosphorylated to the cofactor thiamine diphosphate (ThDP) by a thiamine-phosphate kinase enzyme (ThMP + ATP → ThDP + ADP, EC 2.7.4.16). In most bacteria and eukaryotes, ThMP it is hydrolyzed into thiamine, subsequently pyrophosphorylated into ThDP by the enzyme thiamine diphosphokinase (thiamine + ATP → ThDP + AMP, EC 2.7.6.2).
The biosynthetic pathways of vitamin B1 are regulated by riboswitch, that is, by means of a short strand of RNA capable of directly binding a small target molecule, and as an effect of this modular bond the expression of a gene would occur. If a quantity is present in the cell. sufficient thiamine, this binds to the mRNA of the necessary enzymes and prevents their translation. If it is not present, there is no inhibition and the enzymes necessary for biosynthesis are produced. The specific riboswitch, the riboswitch TPP (or ThDP) , is the only riboswitch identified in both eukaryotic and prokaryotic organisms.
energetic;Note: we are talking about food B1 because, before the enzymatic catalyst action, it is not active and is considered a sort of "transport" of the final molecule.
All organisms use vitamin B1, but as we said it is produced de novo only from bacteria, fungi and plants. Animals have to get it from their diet; therefore, for humans, it is an essential nutrient. Insufficient intake in birds produces a characteristic polyneuritis.
Vitamin B1 phosphates are involved in many cellular processes. The most common form is thiamine pyrophosphate (or diphosphate) (TPP), a coenzyme in the catabolism of sugars and amino acids. In yeasts, TPP is also required in the first phase of alcoholic fermentation.
To date, five natural derivatives of thiamine phosphate are known: thiamine monophosphate (ThMP), thiamine diphosphate (ThDP) - also known as thiamine pyrophosphate (TPP) - thiamine triphosphate (ThTP), l "adenosine thiamine triphosphate (AThTP) and adenosine thiamine triphosphate diphosphate (AThDP).
While the role of the thiamine diphosphate coenzyme is well known and widely described, the non-coenzymatic action of vitamin B1 and its derivatives is less known and probably related to some recently identified proteins that do not exploit the catalytic action. of thiamine diphosphate.