
The intended use is strictly related to the properties of the vegetable or other components which constitute a concentrated source of substances (such as amino acids, vitamins, minerals, fatty acids, fibers, etc.) capable of producing a nutritional and / or physiological effect. In other words, the formulation makes a food supplement suitable to meet specific needs related to your health.
From the point of view of the legislative framework, supplements fall within the macro-category of foods and must, as such, carry certain information on the label for the consumer, established by specific European directives.
For these reasons, the labeling of a food supplement is the subject of particular attention and the presentation of the finished product must meet certain requirements.
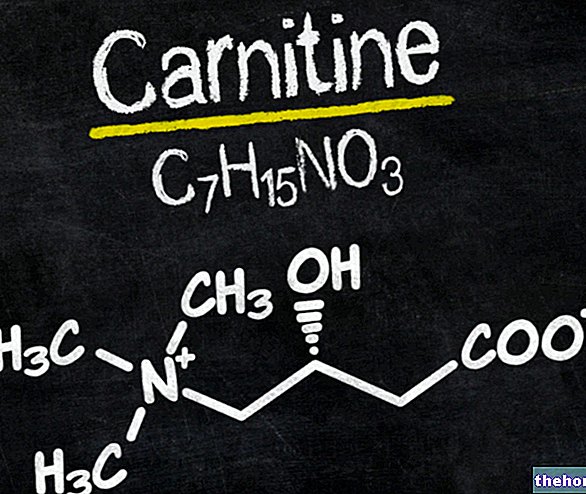
intended to supplement the common diet and which constitute a concentrated source of nutrients, such as vitamins and minerals, or of other substances having a nutritional or physiological effect, in particular, but not exclusively, amino acids, essential fatty acids, fibers and extracts of vegetable origin, both single and multi-compound, in pre-dosed forms ".and a healthy lifestyle;

Furthermore, on the label of food supplements there must NOT be any health claims attributing to the product any properties to prevent, cure or cure a specific pathology.
The labels of food supplements must also include information relating to:
- Who produced it and, if different from the manufacturing company, who made it;
- Lot to which it belongs;
- Expiration date;
- Methods of preservation of the product;
- Format (capsules, tablets, sachets and / or vials) and unit quantity (net weight per unit, i.e. one capsule, one tablet, etc.) and net content of the finished product, expressed in volume units.














.jpg)











