What is Zometa?
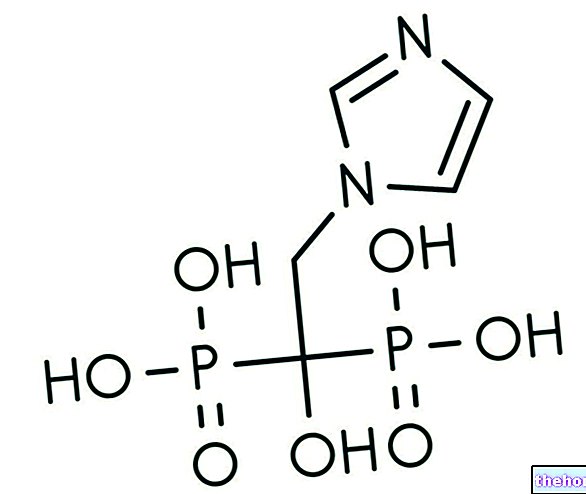
Zometa is a drug containing the active ingredient zoledronic acid, available in powder and solvent and in concentrate, to be diluted to make a solution for infusion (drip into a vein).
What is Zometa used for?
Zometa is indicated to prevent bone complications in patients with advanced cancers affecting the bone. These include fractures, spinal crushing, bone disorders requiring radiation therapy or surgery, and hypercalcaemia (increased level of calcium in the blood).
Zometa can also be used in the treatment of neoplastic hypercalcemia (ie caused by a tumor).
The medicine can only be obtained with a prescription.
How is Zometa used?
Zometa should only be used by physicians experienced in intravenous administration of this type of drug.
The usual dose of Zometa is 4 mg, given as an infusion for at least 15 minutes. If the drug is used to prevent bone complications, the infusion can be repeated every three to four weeks; patients should also take calcium and vitamin D supplements. It is recommended that the dose be reduced in people with bone metastases (spread bone cancer) who report mild to moderate kidney problems. The use of Zometa is not recommended in patients with severe renal insufficiency.
How does Zometa work?
Zoledronic acid, the active ingredient in Zometa, is a bisphosphonate. It inhibits the action of osteoclasts, the body's cells involved in the resorption of bone tissue, with a consequent reduction in bone resorption. The decrease in bone loss. makes bone break less likely, resulting in fracture prevention benefits in patients with bone metastases. People with cancer may have high blood levels of calcium, which is released into the blood from the bones. zoledronic acid promotes the reduction of calcium concentration in the blood.
How has Zometa been studied?
Zometa has been studied in over 3,000 patients with bone metastases in order to verify its effectiveness in preventing bone damage. The medicine was compared with placebo (a dummy treatment) in two studies, while in a third study it was compared with pamidronate (another bisphosphonate). The main measure of effectiveness was the percentage of patients who had at least one new "skeletal event" over 13 months, including any bone complication that needed to be treated with radiotherapy or surgery, any type of fracture or an "onset of vertebral crushing.
The effectiveness of Zometa in patients with cancer hypercalcaemia was investigated in two main studies involving a total of 287 patients, where the medicine was compared with pamidronate. The main measure of effectiveness was the percentage of patients whose calcium levels returned to normal within 10 days of treatment.
What benefit has Zometa shown during the studies?
In patients with bone metastases, the proportion of subjects who developed a new skeletal event was lower with Zometa (33% to 38%) than with placebo (44%). Zometa was also as effective as pamidronate: the percentage of patients experiencing a skeletal event was 44% with Zometa and 46% with pamidronate.
Zometa was more effective than pamidronate in patients with hypercalcaemia. Looking at the results of the two studies together, the percentage of patients with normal calcium levels within 10 days of treatment was 88% with Zometa and 70% with pamidronate, respectively.
What is the risk associated with Zometa?
The most common side effect with Zometa (seen in more than 1 in 10 patients) is hypophosphataemia (decrease in blood phosphate levels). For the full list of side effects reported with Zometa, see the package leaflet.
Zometa should not be used in people who may be hypersensitive (allergic) to zoledronic acid, other bisphosphonates or any of the other ingredients. The medicine should not be used during pregnancy or breastfeeding. As with all bisphosphonates, patients taking Zometa may be at risk for osteonecrosis (bone death) of the jaw.
Why has Zometa been approved?
The Committee for Medicinal Products for Human Use (CHMP) decided that Zometa's benefits are greater than its risks in the prevention of skeletal-related events (pathological fractures, vertebral crushing, radiotherapy or bone surgery, neoplastic hypercalcaemia). in patients with advanced malignant tumors affecting the bone and in the treatment of neoplastic hypercalcemia. The committee recommended the granting of a marketing authorization for Zometa.
Other information about Zometa:
On 20 March 2001, the European Commission granted Novartis Europharm Limited a "Marketing Authorization" for Zometa, valid throughout the European Union. The "Marketing Authorization" was renewed on 20 March 2006.
For the full version of Zometa's EPAR click here.
Last update of this summary: 04-2008
The information on Zometa - zoledronic acid published on this page may be out of date or incomplete. For a correct use of this information, see the Disclaimer and useful information page.