What is Pramipexole Accord - Pramipexole?
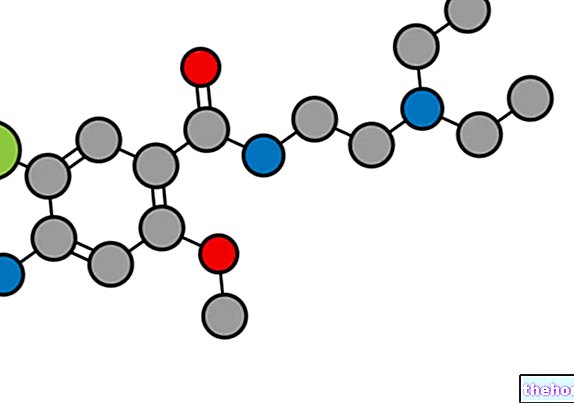
Pramipexole Accord is a medicine that contains the active substance pramipexole. It is available in tablets (0.088; 0.18; 0.35; 0.7 and 1.1 mg).
Pramipexole Accord is a 'generic medicine'. This means that Pramipexole Accord is similar to a 'reference medicine' already authorized in the European Union (EU) called Mirapexin.
What is Pramipexole Accord used for?
Pramipexole Accord is used for treating the symptoms of the following diseases:
Parkinson's disease, which is a progressive mental disorder that causes tremor, slow movement and muscle stiffness; Pramipexole Accord can be used alone or in combination with levodopa (another medicine for Parkinson's disease) at any stage of the disease, including the later stages when the effect of levodopa diminishes;
moderate to severe restless legs syndrome (a disorder that prompts you to move your legs uncontrollably to stop feelings of discomfort, pain or discomfort in the body, especially at night); Pramipexole Accord is used when a specific cause of the disorder cannot be identified.
The medicine can only be obtained with a prescription.
How is Pramipexole Accord used - Pramipexole?
For Parkinson's disease, the starting dosage is one 0.088 mg tablet three times a day. Every five to seven days the dose should be increased until symptoms are controlled without causing undesirable effects that cannot be tolerated. The maximum daily dose corresponds to three 1.1 mg tablets. Pramipexole Accord should be administered less frequently in patients with kidney problems. If for any reason the treatment is stopped, the dose should be decreased gradually.
In the treatment of restless legs syndrome, Pramipexole Accord should be taken once a day, two to three hours before bedtime. The recommended starting dose is 0.088 mg, but, if necessary, it can be increased every 4-7 days to further reduce symptoms, up to a maximum of 0.54 mg. The patient's response and the need for further treatment should be evaluated after three months.
Pramipexole Accord tablets should be taken with water. For more information, see the package leaflet.
How does Pramipexole Accord - Pramipexole work?
The active substance in Pramipexole Accord, pramipexole, is a dopamine agonist (a substance that mimics the action of dopamine). Dopamine is a messenger substance contained in the parts of the brain that control movement and coordination. In patients with Parkinson's disease. there is a loss of the cells that produce dopamine, therefore a reduction in the amount of this substance present in the brain, resulting in a deterioration of the individual's ability to control movements reliably. Pramipexole stimulates the brain as does dopamine, allowing patients to control their movements and reduce the signs and symptoms of Parkinson's disease, including tremors, stiffness and slowed movement.
The mechanism of action of pramipexole in restless legs syndrome is not yet fully understood. It is believed that this syndrome is caused by alterations in the functioning of dopamine in the brain, which can be corrected with pramipexole.
How has Pramipexole Accord been studied - Pramipexole?
As Pramipexole Accord is a generic medicine, the studies have been limited to evidence designed to show that the medicine is bioequivalent to the reference medicine Mirapexin.Two medicines are bioequivalent when they produce the same levels of the active substance in the body.
What are the benefits and risks of Pramipexole Accord - Pramipexole?
Because Pramipexole Accord is a generic medicine and is bioequivalent to the reference medicine, its benefits and risks are taken as being the same as the reference medicine's.
Why has Pramipexole Accord - Pramipexole been approved?
The CHMP concluded that, in accordance with EU requirements, Pramipexole Accord has been shown to have comparable quality and to be bioequivalent / comparable to Mirapexin. It is therefore the CHMP's view that, as in the case of Mirapexin, the benefits outweigh the identified risks. The Committee therefore recommended the granting of marketing authorization for Pramipexole Accord.
Other information about Pramipexole Accord - Pramipexole
On 30 September 2011, the European Commission issued a "Marketing Authorization" for Pramipexole Accord, valid throughout the European Union.
For more information about Pramipexole Accord therapy, read the package leaflet (included with the EPAR) or contact your doctor or pharmacist.
The full EPAR version of the reference medicine can be found on the Agency's website.
Last update of this summary: 08/2011.
The information on Pramipexole Accord - Pramipexole published on this page may be out of date or incomplete. For a correct use of this information, see the Disclaimer and useful information page.