What is Kuvan?
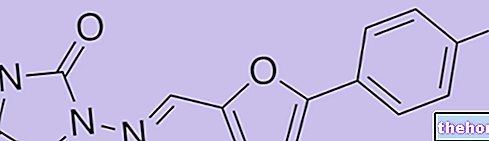
Kuvan is a medicine that contains the active substance sapropterin dihydrochloride. It is available as light yellow soluble tablets (100 mg).
What is Kuvan used for?
Kuvan is used to treat hyperphenylalaninemia (HPA, high levels of phenylalanine in the blood) in patients with the genetic disorders phenylketonuria (PKU) or tetrahydrobiopterin (BH4) deficiency. Patients with these disorders cannot convert the amino acid. phenylalanine (found in food proteins) to tyrosine (another amino acid). This causes a buildup of phenylalanine in the blood which can cause brain and nervous system problems.
Kuvan can be used in adults and children. Kuvan is indicated for use in children with HPA due to PKU who are at least four years of age. The medicine has not been studied in children with HPA due to PKU under the age of four. Kuvan can be used in children of all ages with HPA due to BH4 deficiency.
Kuvan treatment should only be continued in patients who respond adequately to the medicine.
Because the number of patients with HPA is low, the disease is considered 'rare' and Kuvan was designated an 'orphan medicine' (a medicine used in rare diseases) on 8 June 2004.
The medicine can only be obtained with a prescription.
How is Kuvan used?
Kuvan therapy should be initiated and supervised by a physician experienced in the treatment of PKU and BH4 deficiency. The amount of phenylalanine and protein in the patient's diet should be monitored to ensure that blood phenylalanine levels and nutritional balance are controlled. Kuvan is intended for long-term use.
The dose of Kuvan depends on the patient's weight. Patients with PKU should start with 10 mg per kilogram of body weight once daily and those with BH4 deficiency should start with 2 - 5 mg / kg once daily. After one week, the dose can be adjusted up to 20 mg / kg once daily if the patient has not responded to treatment. A satisfactory response s "means a reduction in blood phenylalanine levels by at least 30% or to a level determined by the physician. If this response is obtained after one month, the patient is classified as" responsive "and can continue to take Kuvan. .
Kuvan is taken together with a meal at the same time each day, preferably in the morning. The tablets dissolve in a glass of water before the patient drinks the solution. For some patients with BH4 deficiency, the dose sometimes needs to be divided into two or three doses throughout the day for best effect. Kuvan should be used with caution in patients over the age of 65 and in patients with liver or kidney problems.
How does Kuvan work?
The high levels of phenylalanine in the blood are due to a problem with the conversion of phenylalanine to tyrosine by the "phenylalanine hydroxylase" enzyme. Patients with PKU have a defective version of the enzyme and patients with BH4 deficiency have low levels of BH4. a "cofactor" that serves the enzyme to function properly. Kuvan's active ingredient, sapropterin dihydrochloride, is a synthetic copy of BH4. In PKU, it acts by enhancing the activity of the defective enzyme, and in BH4 deficiency it replaces the cofactor This helps restore the enzyme's ability to convert phenylalanine to tyrosine, reducing phenylalanine levels in the blood.
How has Kuvan been studied?
Kuvan's effects were first tested in experimental models before being studied in humans.
For the treatment of PKU patients, Kuvan has been studied in two main studies comparing Kuvan with placebo. All the patients participating in the studies had shown a response to a first eight-day course of Kuvan but had spent a period of at least one week without the medicine before the studies began.
The first study included 89 patients aged eight years and over who did not follow a strict diet. The main measure of effectiveness was the reduction in blood phenylalanine levels over six weeks.
The second study included 46 children between the ages of four and 12 who were dieting with a controlled level of phenylalanine. From the third week of therapy, the diet was adjusted every two weeks based on blood phenylalanine levels. The main measure of effectiveness was the change in the amount of phenylalanine that the children could get through food while keeping the blood phenylalanine at desired levels. The study lasted 10 weeks.
For the treatment of patients with BH4 deficiency, the company presented the results of three studies available in the published literature on sapropterin dihydrochloride. One of these studies included 16 patients treated for an average of 15.5 months.
What benefit has Kuvan shown during the studies?
For the treatment of PKU, Kuvan was more effective than placebo. In the first study, blood phenylalanine levels were around 867 "micromoles per liter" at the start of the study. Normal levels are around 60 micromoles per liter in people without PKU. After six weeks, phenylalanine levels had decreased. 236 micromoles per liter in the patients taking Kuvan and increased by 3 micromoles per liter in the patients taking placebo. In the second study, children taking Kuvan could take an average of 17.5 mg more phenylalanine through their diet. per pound of body weight every day after 10 weeks, compared to 3.3 mg more for children taking placebo.
In studies of patients with BH4 deficiency, patients showed improvement in blood phenylalanine levels and other disease markers when taking sapropterin dihydrochloride.
What is the risk associated with Kuvan?
The most common side effects with Kuvan (seen in more than 1 in 10 patients) are headache and runny nose (runny nose). For the full list of side effects reported with Kuvan, see the Package Leaflet.
Kuvan must not be used in people who may be hypersensitive (allergic) to sapropterin dihydrochloride or any of the other substances.
Why has Kuvan been approved?
The Committee for Medicinal Products for Human Use (CHMP) decided that Kuvan's benefits are greater than its risks for the treatment of HPA in adult and pediatric patients with PKU and BH4 deficiency who have been responsive to this therapy. The Committee recommended the granting of marketing authorization for Kuvan.
Other information about Kuvan:
On 2 December 2008, the European Commission granted Merck KGaA a "Marketing Authorization" for Kuvan, valid throughout the European Union.
For the summary of the opinion of the Committee for Orphan Medicinal Products for Kuvan, click here.
For the full version of Kuvan's EPAR, click here.
Last update of this summary: 10-2008.
The information on Kuvan - sapropterin dihydrochloride published on this page may be out of date or incomplete. For a correct use of this information, see the Disclaimer and useful information page.